Names and Formulas for Ionic Compounds Formula Unit









- Slides: 13

Names and Formulas for Ionic Compounds

Formula Unit Review Ø In ionic compounds, we must balance charges to form a neutral compound Ø To do that, we “crisscross the charges” similar to find the least common denominator in a fraction Ø Always write the cation first (metal) followed by the anion (nonmetal) Ø Ca and N, what will the formula be?

Practice ØAl and S ØMg and O ØNa and N

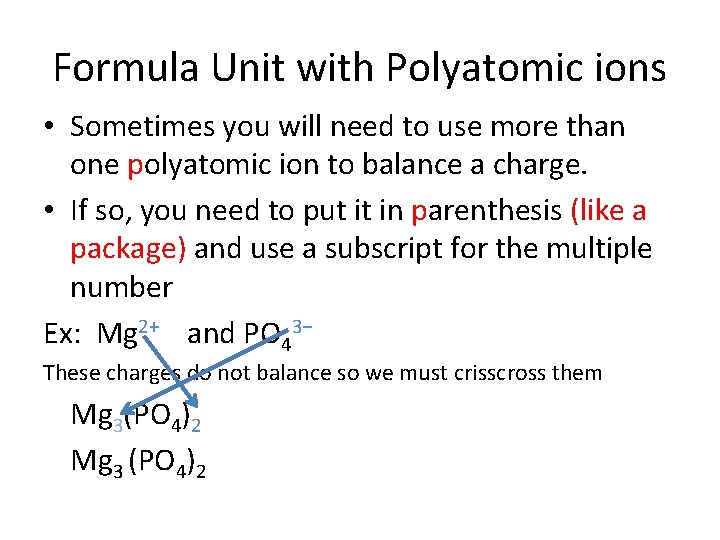
Formula Unit with Polyatomic ions • Sometimes you will need to use more than one polyatomic ion to balance a charge. • If so, you need to put it in parenthesis (like a package) and use a subscript for the multiple number Ex: Mg 2+ and PO 43− These charges do not balance so we must crisscross them Mg 3(PO 4)2 Mg 3 (PO 4)2

Naming Ca”t”ions • Monatomic cation name stays the same – Sodium, Na goes to sodium ion, Na+ – Calcium, Ca goes to calcium ion, Ca 2+ • Polyatomic cation - look up in chart – NH 4+ ammonium

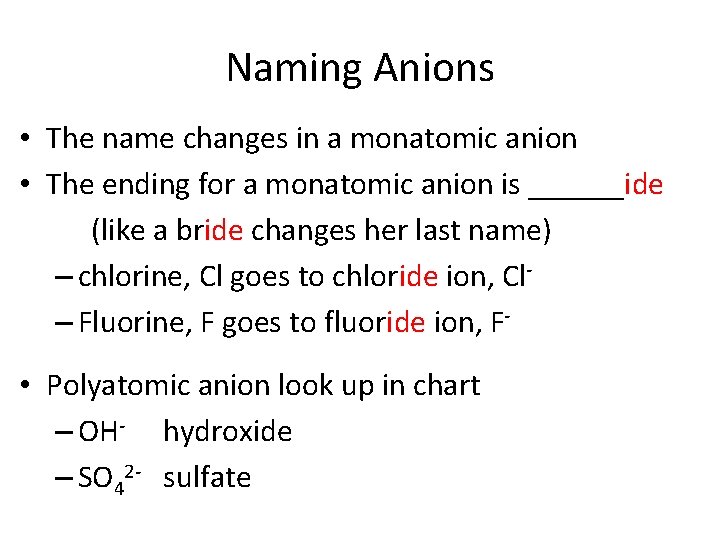
Naming Anions • The name changes in a monatomic anion • The ending for a monatomic anion is ______ide (like a bride changes her last name) – chlorine, Cl goes to chloride ion, Cl– Fluorine, F goes to fluoride ion, F • Polyatomic anion look up in chart – OH- hydroxide – SO 42 - sulfate

Naming ionic compounds • Name the cation first and then the anion second (+ then -) • Example: Cs. F – Monatomic cations use the element name • Cs+ = cesium ion – Monatomic anions – change the ending to ide • F - = fluoride ion cesium fluoride

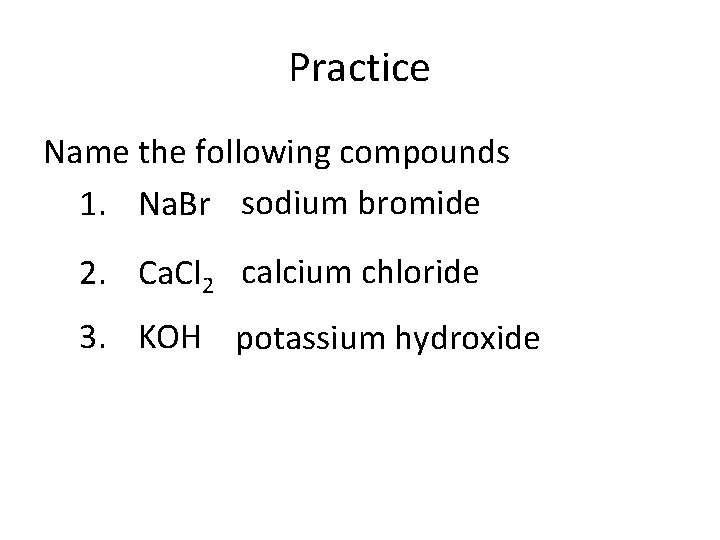
Practice Name the following compounds 1. Na. Br sodium bromide 2. Ca. Cl 2 calcium chloride 3. KOH potassium hydroxide

Will these form an Ionic Compound? If so what will the formula name be? 1. 2. 3. 4. 5. Mg Al Mg Na C Al S O N S No Yes two metals aluminum sulfide Yes magnesium oxide Yes sodium nitride No two non-metals

Transition Metal Ions • Transitions metals will form cations • Some transition metals only have one ionic charge – Silver (Ag+), Cadmium (Cd 2+), Zinc (Zn 2+) • Some transition metals can take on more than one charge. – Copper (Cu+, Cu 2+) • Charge is determined from the number of electrons lost

Naming Transition Metal Ions • If a transition metal has more than one charge, the charge is written as a Roman numeral in parenthesis • Some metals need a middle name • Fe (iron) has more than one charge: 2+ and 3+ – Fe 2+ – Fe 3+ iron(II) iron(III) • If the transition metal only has one charge, no Roman numeral is needed

Naming Ionic Compounds with Transition Metals Given: Fe 2 O 3 • First find the charges of the ions • Then name the cation followed by the anion – Iron(III)oxide • Try this one: Fe 3 P 2 • the charge on iron is a 2+ • iron(II) phosphide

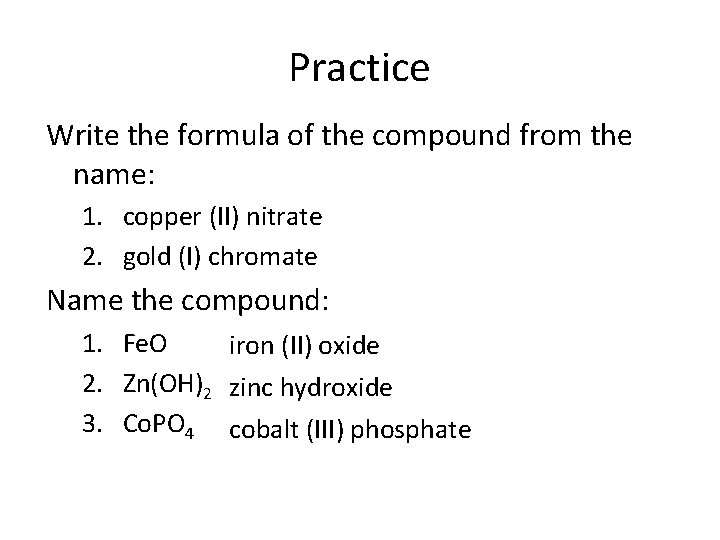
Practice Write the formula of the compound from the name: 1. copper (II) nitrate 2. gold (I) chromate Name the compound: 1. Fe. O iron (II) oxide 2. Zn(OH)2 zinc hydroxide 3. Co. PO 4 cobalt (III) phosphate