Name the following compounds Ca CO 3 calcium









- Slides: 14

Name the following compounds: Ca. CO 3 calcium carbonate Na 2 SO 4 sodium sulfate barium chloride lithium bromide Ba. Cl 2 Li. Br Ag. NO 3 HCl silver nitrate nitric acid hydrochloric acid

4. 2 Tests for Negative Ions Objective: to explain how to use chemical tests to identify the negative ions within different substances Outcomes: All: I can identify the symbols for carbonate, sulfate and halides. (D/E) Most: I can describe how to carry out and state the result for tests for these 3 negative ions. (C) Some: I can interpret results of chemical tests independently. (A/B) Keywords: negative ion, precipitate, ionic equation Homework: Complete revision card for a C 3 topic of your choice Due: TODAY!

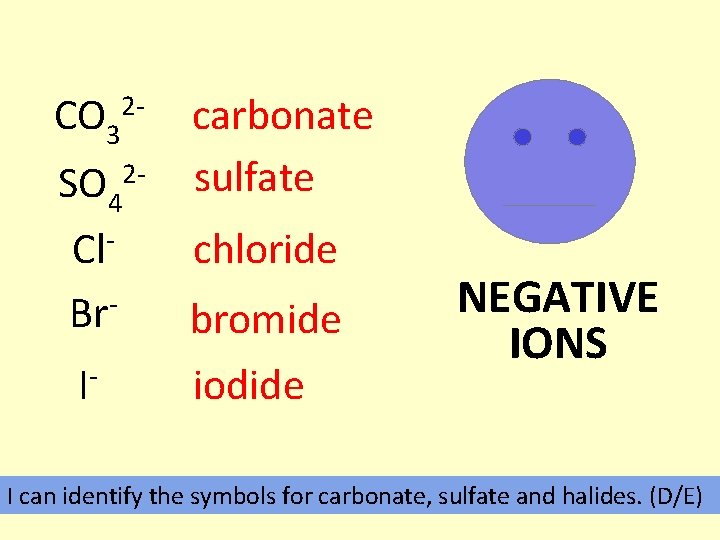
CO 3 2 - SO 4 2 - carbonate sulfate Cl. Br- bromide I- iodide chloride NEGATIVE IONS I can identify the symbols for carbonate, sulfate and halides. (D/E)

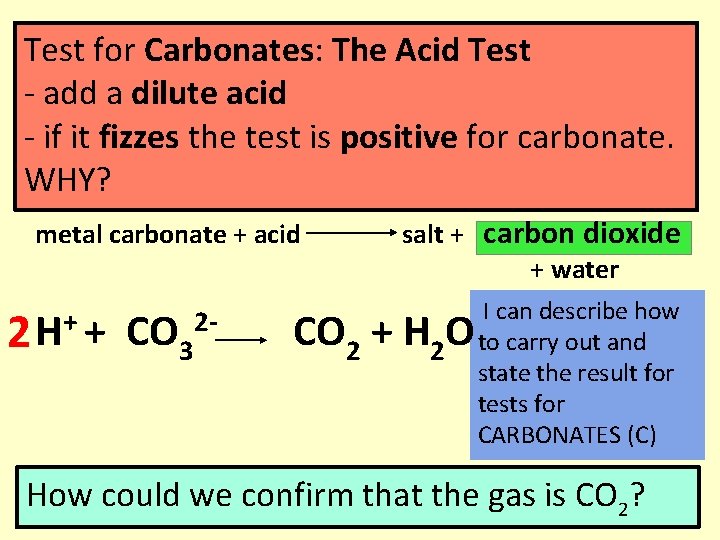
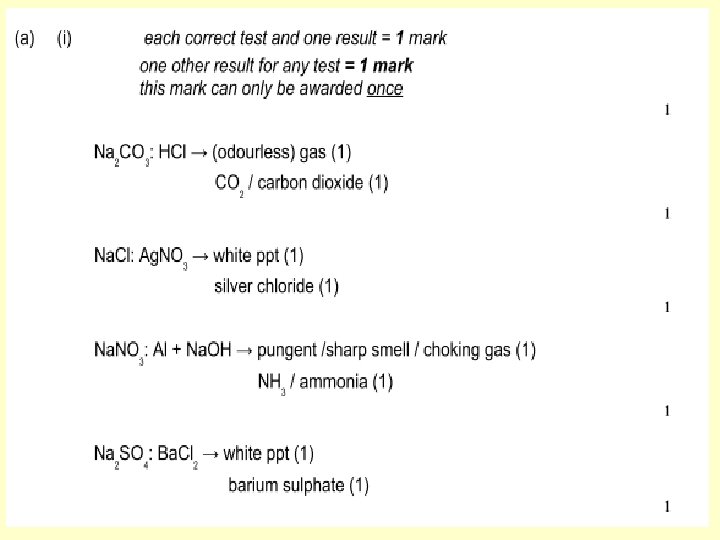
Test for Carbonates: The Acid Test - add a dilute acid - if it fizzes the test is positive for carbonate. WHY? metal carbonate + acid 2 + H + CO 3 2 - salt + carbon dioxide + water CO 2 + H 2 O I can describe how to carry out and state the result for tests for CARBONATES (C) How could we confirm that the gas is CO 2?

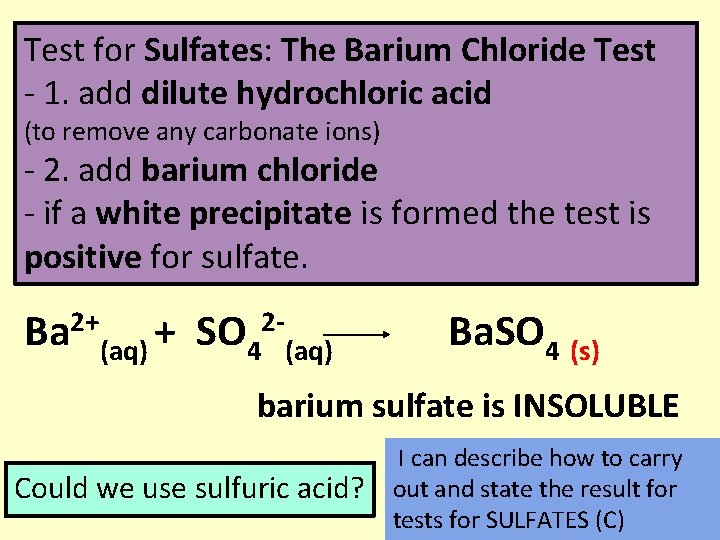
Test for Sulfates: The Barium Chloride Test - 1. add dilute hydrochloric acid (to remove any carbonate ions) - 2. add barium chloride - if a white precipitate is formed the test is positive for sulfate. 2+ Ba (aq) + SO 4 2 - (aq) Ba. SO 4 (s) barium sulfate is INSOLUBLE Could we use sulfuric acid? I can describe how to carry out and state the result for tests for SULFATES (C)

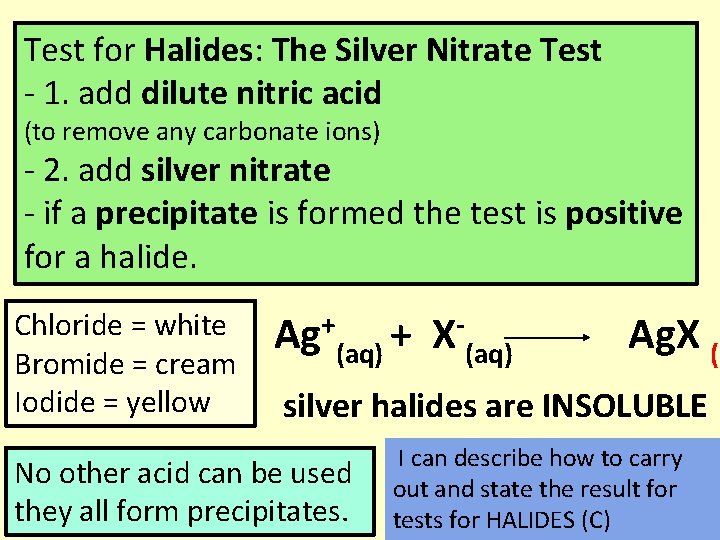
Test for Halides: The Silver Nitrate Test - 1. add dilute nitric acid (to remove any carbonate ions) - 2. add silver nitrate - if a precipitate is formed the test is positive for a halide. Chloride = white Bromide = cream Iodide = yellow + Ag (aq) + X (aq) Ag. X (s silver halides are INSOLUBLE No other acid can be used they all form precipitates. I can describe how to carry out and state the result for tests for HALIDES (C)

MYSTERY CHEMICAL What am I? . . . Using the tests for positive and negative ions that you have learned identify the unknown substances! I can interpret results of chemical tests independently. (A/B)

Note: this is levelled as a HARD question (i. e. the kind to expect in your exam)






4. 2 Tests for Negative Ions Objective: to explain how to use chemical tests to identify the negative ions within different substances Outcomes: All: I can identify the symbols for carbonate, sulfate and halides. (D/E) Most: I can describe how to carry out and state the result for tests for these 3 negative ions. (C) Some: I can interpret results of chemical tests independently. (A/B) Keywords: negative ion, precipitate, ionic equation Homework: Complete revision card for a C 3 topic of your choice Due: TODAY!