Name Bunsen burner Conical flask 1 Scientific equipment

Name Bunsen burner Conical flask 1. Scientific equipment Use Heating by burning a gas To contain reactions that effervesce. Beaker To hold, pour and heat liquids Measuring To measure cylinder precise volume of liquid Evaporatin To heat and g basin evaporate liquids Year 7 – The Knowledge – Science – Autumn 1 - Particles Name Stopwatch Tongs Thermome ter Safety Goggles Tripod Gauze 2. Bunsen burner Different flames: Use To measure time To hold hot things (not test tubes) To measure temperature To protect your eyes To hold a beaker above a Bunsen burner Used to support a beaker 3. The particle model Collar open= Blue flame- used to heat substances Temperature= 500 degrees Collar closed= Yellow flame: used as a safety flame. Temperature= 300 degrees 5. Changes of state 7. Diffusion Sodium- Na Magnesium- Mg Aluminium- Al Silicon- Si Phosphorus- P Sulfur- S Chlorine- Cl Argon- Ar Potassium- K Calcium- Ca Particles Freezing Liquid to solid Lose energy, only vibrate on the spot. Bonds form Melting Solid to liquid Get more energy, move faster. Bonds begin to break. Particles move out of regular pattern Evaporating Liquid to gas Get more energy, move faster. Bonds begin to break Condensing Gas to liquid Lose energy, move slower. Bonds begin to form 8. Metals and non-metals have opposite properties to each other: Particles: The particles stay the same size- it is the space between the particles that changes: When chemicals, like the smell of perfume or burning toast, are let loose in a room, the particles mix with the air particles. Diffusion is the spreading out of particles Gas: Diffusion is very quick in gases as the particles in a gas move quickly Liquid: Diffusion can happen in liquids but slower, this is because the particles in liquids can move around each other Solid: Diffusion can not happen in solids as the particles cannot move, they only vibrate on the spot (b) Boiling point: the temperature at which a material changes from a liquid to a gas (boils). The boiling point of water is 100 degrees. (c) Atom: tiny particles- make up everything, including you! HCl- Hydrochloric acid Cu. SO 4 - Copper sulfate (d) Element: the same type of atom H 20 - Water Na. OH- Sodium e. g. Gold contains only gold atoms Mg. O- Magnesium hydroxide Na. Cl- Sodium Chloride(e) Molecule: elements that move CO 2 - Carbon dioxide CH 4 - Methane around in groups of atoms e. g. O 2 SO 2 - Sulfur dioxide NH 3 - Ammonia (f) Compound: two or more elements chemically bonded together Change of state Contraction: Substances contract (decrease in size) when they get cooler (a) Melting point: the temperature at which a material changes from a solid to a liquid (melts). The melting point of water is 0 degrees. Top 10 compounds Keyword 6. Expansion and contraction Expansion: Substances expand (increase in size) when they get warmer Uses: Thermometers work in this way- the liquid expands and rises up the tube when it gets hotter Particles: The particles stay the same size- it is the space between the particles that changes: Hydrogen-H Helium- He Lithium - Li Beryllium- Be Boron- B Carbon- C Nitrogen- N Oxygen- O Fluorine- F Neon- Ne Bonds Strong Weak No bonds 4. Hazard symbols Keyword definitions Top 20 elements (g) Mixture: two or more types of atoms mixed together, no bonds 9. Elements, compounds and mixtures Elements: Each element is given its own chemical symbol (look up the periodic table!) e. g H for hydrogen, O for oxygen Every chemical symbol starts with a capital letter, with the second letter written in lower case e. g. Mg is the symbol for magnesium (not MG, mg, m. G) Compounds: compounds contain two ore more types of atoms that are chemically bonded. See below for 2 examples:
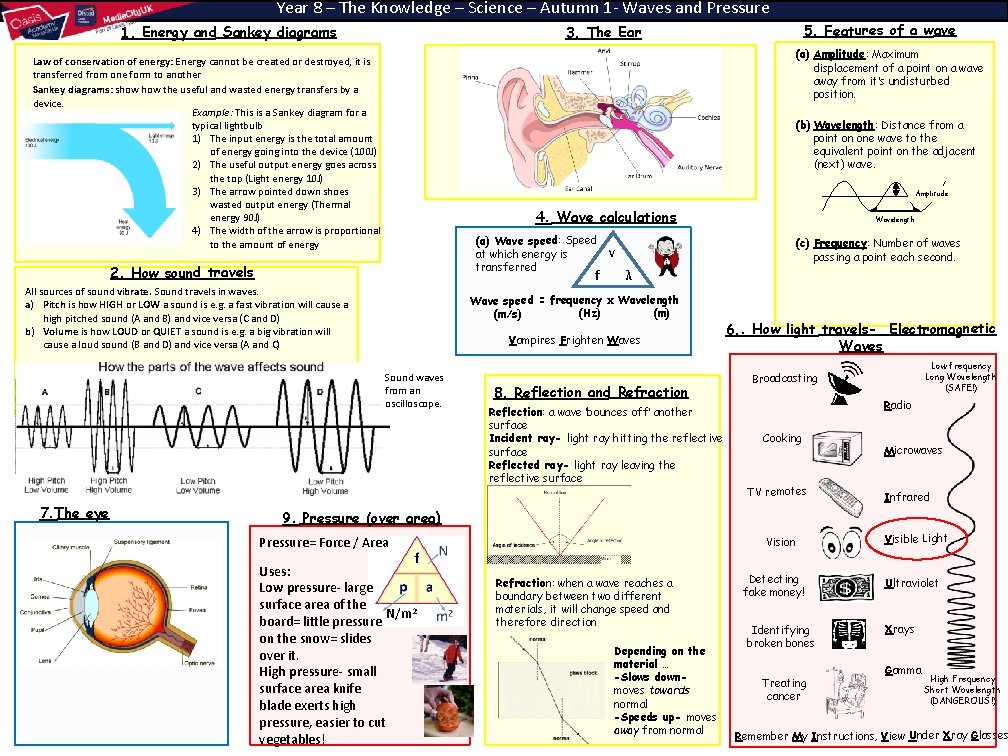
Year 8 – The Knowledge – Science – Autumn 1 - Waves and Pressure 1. Energy and Sankey diagrams (a) Amplitude: Maximum displacement of a point on a wave away from it’s undisturbed position. Law of conservation of energy: Energy cannot be created or destroyed, it is transferred from one form to another Sankey diagrams: show the useful and wasted energy transfers by a device. Example: This is a Sankey diagram for a typical lightbulb 1) The input energy is the total amount of energy going into the device (100 J) 2) The useful output energy goes across the top (Light energy 10 J) 3) The arrow pointed down shoes wasted output energy (Thermal energy 90 J) 4) The width of the arrow is proportional to the amount of energy (b) Wavelength: Distance from a point on one wave to the equivalent point on the adjacent (next) wave. Amplitude 4. Wave calculations (a) Wave speed: Speed at which energy is transferred 2. How sound travels f All sources of sound vibrate. Sound travels in waves. a) Pitch is how HIGH or LOW a sound is e. g. a fast vibration will cause a high pitched sound (A and B) and vice versa (C and D) b) Volume is how LOUD or QUIET a sound is e. g. a big vibration will cause a loud sound (B and D) and vice versa (A and C) Wavelength (c) Frequency: Number of waves passing a point each second. v λ Wave speed = frequency x Wavelength (m) (Hz) (m/s) Vampires Frighten Waves Sound waves from an oscilloscope. 7. The eye 5. Features of a wave 3. The Ear 8. Reflection and Refraction Reflection: a wave ‘bounces off’ another surface Incident ray- light ray hitting the reflective surface Reflected ray- light ray leaving the reflective surface 6. . How light travels- Electromagnetic Waves Low frequency Long Wavelength (SAFE!) Broadcasting Radio Cooking TV remotes Microwaves Infrared 9. Pressure (over area) Pressure= Force / Area Uses: Low pressure- large surface area of the N/m 2 board= little pressure on the snow= slides over it. High pressure- small surface area knife blade exerts high pressure, easier to cut vegetables! Vision Refraction: when a wave reaches a boundary between two different materials, it will change speed and therefore direction Depending on the material … -Slows downmoves towards normal -Speeds up- moves away from normal Detecting fake money! Identifying broken bones Treating cancer Visible Light Ultraviolet Xrays Gamma High Frequency Short Wavelength (DANGEROUS!) Remember My Instructions, View Under Xray Glasses

Angle Properties Year 9 – The Knowledge – Autumn 1 – Chemistry Fundamentals 2. Atoms, Elements, Compounds and Mixtures 1. States of Matter 3 states of matter: solid, liquid, gas. Particle model explains how particles are arranged and how they move. melting freezing boiling condensing Melting point: temperature at which a pure substance melts and freezes. Boiling point: temperature at which a pure substance boils and condenses. The stronger the forces between particles, the higher the melting point and boiling point. 4. Atomic Theories – Timeline Due to new experimental evidence, the scientific model of the atom has changed over time. Order of atomic theories and why they were updated: 1. Before the discovery of the electron - Dalton Model: atoms were thought to be tiny spheres that could not be divided. 3. After alpha particle scattering experiment - Rutherford Model: mass of atom is concentrated at the centre (nucleus). Nucleus is positively charged. 5. Protons, Neutrons, Electrons In an atom, the number of electrons is equal to the number of protons in the nucleus. There is no overall charge of an atom. Radius of atom: 0. 1 nm (1 x 10 -10 m) Radius of nucleus: 1 x 10 -14 m (1/10 000 of radius of atom) Almost all of the mass of an atom is in the nucleus (i. e. protons and neutrons are MUCH heavier than electrons). Name of particle Relative Charge Relative Mass Proton +1 1 Neutron 0 1 Electron -1 Very small (“negligible”) NB: atomic number is always smaller than the mass number 4. After Niels Bohr made some theoretical calculations that agreed with experimental observation - Bohr Model: In addition to the Rutherford model, electrons orbit the nucleus at specific distances. The atomic mass number Z and the mass number A can be used to determine the number of protons, neutrons and electrons in an atom: Number of protons = atomic number = Z Number of neutrons = mass number – atomic number = A - Z Number of electrons = atomic number = Z Positively charged, made up of protons 8. Ar and Mr 5. Later experiments led to idea that the positive charge of the nucleus cold be subdivided into a whole number of smaller particles. Each of these smaller particles has the same charge. These particles are called protons. Nuclear Model 6. James Chadwick’s experimental work provided evidence of neutrons inside the nucleus as well as protons. The model that we used today we call the nuclear model. Positively charged, made up of protons and neutrons 3. Separation of Compounds and Mixtures Compounds can only be separated into elements by chemical reactions. Mixtures are separated by physical processes no chemical reaction is involved, no new substance are made. Separation technique Used to separate Chemical or physical Chemical reaction Compounds Chemical Filtration Mixture of liquid and insoluble solid Physical Crystallisation Mixture of liquid and soluble solid (solid retrieved) Physical Simple distillation Mixture of liquid and soluble solid (solvent/liquid retrieved) Physical Fractional distillation Mixture of two or more liquids that have different boiling points Physical Chromatography Mixture of soluble coloured compounds in solution Physical 6. Electronic Configuration Atomic number: number of protons in an atom of an element. NB: all atoms of a particular element have the same number of protons; atoms of different elements have different number of protons. Mass number: sum of the number of protons and neutrons in an atom. The atomic number and mass number for each element can be found on the periodic table: 2. After discovery of the electron - Plum Pudding Model: Atom is a ball of positive charge with electrons embedded in it. 7. Isotopes and Ions All substances are made from atoms. Atom: smallest part of an element. Element: substance that contains only one type of atom (e. g. sodium, Na). Compound: substance that contains two or more elements chemically combined (e. g. sodium chloride, Na. Cl). NB: chemical property of compound is different to chemical property of each element in the compound. Naming: ‘-ine’ – halogen element (e. g. chlorine, Cl); ‘-ide’ – compound without oxygen (e. g. calcium chloride Ca. Cl 2); ‘-ate’ – compound with oxygen (calcium carbonate, Ca. CO 3) Mixture: two or more elements or compounds not chemically combined together. NB: chemical properties of each substance in the mixture are unchanged. Relative atomic mass (Ar): average of the atomic masses of all the different isotopes in a sample taking into account the proportion of each isotope in the sample. Ar=Total mass of all atoms of an element Total number of atoms of that element Mass numbers, A, given in periodic table are Ar for all naturally occurring isotopes on Earth. Relative formula mass (Mr): sum of the Ar of the atoms shown in the formula of compound. e. g. Mr of H 2 O: Ar (H) = 1, Ar (O) = 16 Mr (H 2 O) = 1 + 16 = 18. 10. Periodic Table A. Layout Elements are arranged in order of atomic number, starting with the lowest. Groups: columns of the periodic table. Tells us the number of electrons in their outer shell (e. g. group 1 elements have one electron), they have similar chemical properties. Periods: rows of the periodic table. B. History Elements were arranged in order of increasing atomic mass and not all the elements had been discovered resulting in some elements being placed in inappropriate groups. Mendeleev overcame this problem by leaving gaps for elements that he thought would be discovered, and in places switched the order of elements so that all elements in each group had similar properties. After discovery of protons, neutrons and electrons, it turned out Mendeleev had ordered atoms in order of atomic number. Electronic configuration for sodium as a diagram 9. Gas Tests Gas Properties Test Observation Hydrogen (H 2) Colourless gas Burning splint Burns rapidly with a pop sound Oxygen (O 2) Colourless gas Glowing splint Splint relights Carbon dioxide (CO 2) Colourless gas Bubble or shake through limewater Limewater turns milky (cloudy) Chlorine (Cl 2) Green gas Damp litmus paper Paper is bleached and turns white C. Metals and Non-Metals Metal (left of bold line, excluding hydrogen): element that reacts to form a positive ion Non-metal (right of bold line): element that reacts to form a negative ion. Alloy: Mixture of two or more elements, where at least one element is a metal. In pure metals, atoms are arranged in layers, which allows metals to be bent and shaped. Pure metals are too soft for many uses. Alloys contain atoms of different sizes, which distort the regular arrangements of atoms making it more difficult for the layers to slide over each otherefore are harder. Metals Non-metals High melting point Low melting point Good thermal conductors Poor thermal conductors (insulators) Good electrical conductors Poor electrical conductors (insulators) Ductile (can be drawn into wires) Not ductile Malleable Not malleable Most are solids at room temperature Most are liquids or gases at room temperature

Angle Properties Year 9 – The Knowledge – Science – Autumn 1 – Chemistry Fundamentals E. Group 1 – Alkali Metals D. Group 0 – Noble Gases Properties: non-metal, unreactive (do not easily form molecules), gaseous at room temperature Electronic configuration: have a full outer shell Group trend: boiling points increase down group Why noble gases are so unreactive/inert: They have a full outer shall of electrons as shown in the electronic configuration diagrams. 11 a. Corrosion (triple only) Called alkali metals because when the metal hydroxide is dissolved in water, the solution is alkaline. Properties: highly reactive, low melting and boiling points, low density, shiny when cut, soft Electronic configuration: one electron in outer shell Group trends: reactivity increases down the group; melting and boiling points decrease down the group Reactions: Lose the electron in outer shell to form positive ions with charge +1. Why reactivity increases down the group: Larger the atom more electrons more shells outer shell further from (positive) nucleus. The larger the distance between (negative) outer shell electron and the (positive) nucleus, the weaker the electrostatic attractive force easier to lose the outer electron more reactive 1 2 Corrosion: destruction of materials by chemical reactions with substances in the environment. Example: Rusting of Iron. Rusting occurs only when both water and air (oxygen) are present. If only air or only water are present, then iron will not rust. 11 b. Corrosion Prevention (triple only) 3 Test tube 1: Air and water present – iron nail rusts Test tube 2: Water only Oil is used as a barrier to prevent air from getting to water and nail – iron nail does not rust Test tube 3: Air only : Calcium chloride is used to absorb any water – iron nail does not rust Corrosion can be prevented by applying a coating that acts as a barrier. Examples: • Greasing • Painting • Electroplating Aluminium reacts with oxygen in the air to form aluminium oxide – this oxide coating acts as a barrier to prevent further corrosion. Sacrificial protection: using a more reactive metal as a coating the more reactive metal will corrode instead. Example: iron is galvanised (coated in zinc). Even if a scratch occurs, the corrosion of iron will be slow because zinc is more reactive. F. Group 7 – Halogens Properties: non-metal, highly reactive, diatomic. Electronic configuration: seven electrons in outer shell Group trends: reactivity decreases down the group, melting and boiling points increase down the group. Reactions: Gain one electron in their outer shell to form negative ions with charge -1. React with metals to produce ionic salts (metal halides). A more reactive halogen will displace a less reactive halogen from an aqueous solution of its salt. Why reactivity decreases down the group: Smaller the atom fewer electrons less shells outer shell closer to (positive) nucleus. Halogens gain one electron when forming compounds. The closer the outer shell is to the (positive) nucleus, the stronger the electrostatic attractive force is between the nucleus and the electron being added to the outer shell. 12. Alloys as Useful Materials By varying both the types of metals in an alloy and their ratio, alloys of different properties can be made. Alloy Made up of Property Use Bronze Copper and tin Bright gold colour Coins, statues, decorative objects Brass Copper and zinc Hard-wearing, resistant to corrosion Water taps and door fittings Gold alloys Gold with silver, copper and/or zinc Unreactive Very good conductor of electricity Jewellery Electrical connections on circuit boards ~0. 25% carbon and other metals ~2. 5% carbon and other metals Chromium and nickel Hard and strong -car body panels Soft and easily shaped -cutting tools Hard and resistant to corrosion -cutlery and sinks Aluminium and other metals Low density and high strength Making aeroplanes Steel -low carbon steel -high carbon steel -stainless steel Aluminium alloy
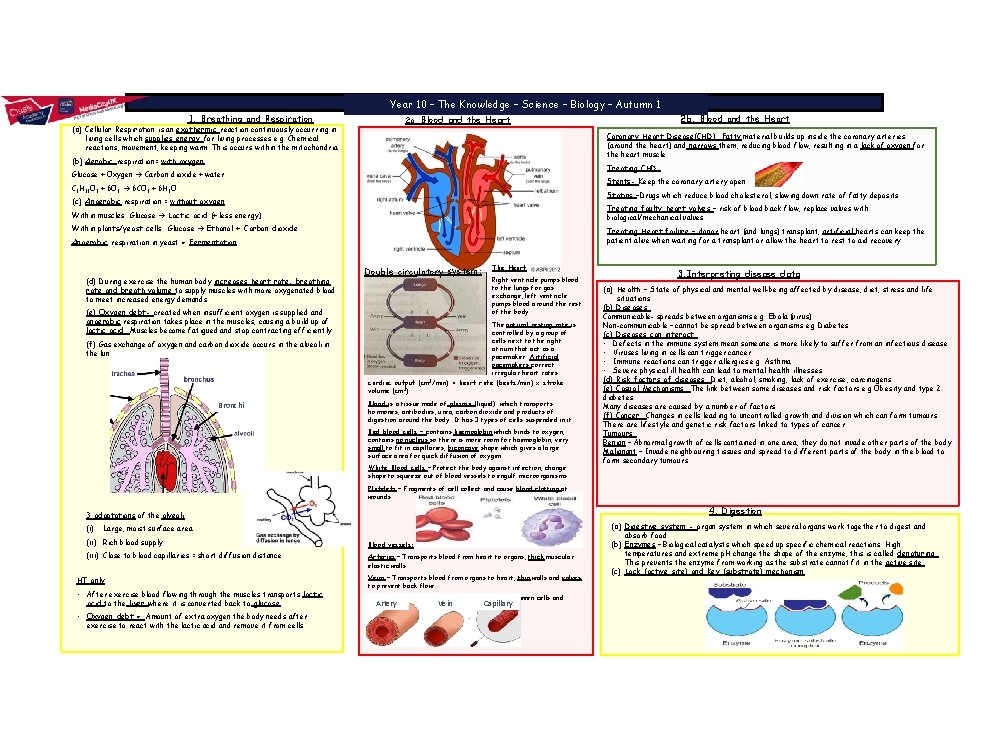
Angle Properties Year 10 – The Knowledge – Science – Biology – Autumn 1 1. Breathing and Respiration (a) Cellular Respiration is an exothermic reaction continuously occurring in 2 a. Coronary Heart Disease(CHD): Fatty material builds up inside the coronary arteries (around the heart) and narrows them, reducing blood flow, resulting in a lack of oxygen for the heart muscle. living cells which supplies energy for living processes e. g. Chemical reactions, movement, keeping warm. This occurs within the mitochondria (b) Aerobic respiration= with oxygen Treating CHD: Glucose + Oxygen Carbon dioxide + water Stents- Keep the coronary artery open C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O Statins –Drugs which reduce blood cholesterol, Le Chaslowing down rate of fatty deposits. (c) Anaerobic respiration = without oxygen Treating faulty heart valves – risk of blood back flow, replace valves with biological/mechanical valves. Within muscles: Glucose Lactic acid (+ less energy) Within plants/yeast cells: Glucose Ethanol + Carbon dioxide Treating Heart failure – donor heart (and lungs) transplant, artificial hearts can keep the patient alive when waiting for a transplant or allow the heart to rest to aid recovery. Anaerobic respiration in yeast = Fermentation (d) During exercise the human body increases heart rate, breathing rate and breath volume to supply muscles with more oxygenated blood to meet increased energy demands. (e) Oxygen debt- created when insufficient oxygen is supplied anaerobic respiration takes place in the muscles, causing a build up of lactic acid. Muscles become fatigued and stop contracting efficiently. (f) Gas exchange of oxygen and carbon dioxide occurs in the alveoli in the lungs. Bronchi 2 b. Blood and the Heart Double circulatory system: The Heart Right ventricle pumps blood to the lungs for gas exchange, left ventricle pumps blood around the rest of the body. The natural resting rate is controlled by a group of cells next to the right atrium that act as a pacemaker. Artificial pacemakers correct irregular heart rates. cardiac output (cm 3/min) = heart rate (beats/min) x stroke volume (cm 3) Blood is a tissue made of plasma (liquid) which transports hormones, antibodies, urea, carbon dioxide and products of digestion around the body. It has 3 types of cells suspended in it: Red blood cells – contains haemoglobin which binds to oxygen, contains no nucleus so there is more room for haemoglobin, very small to fit in capillaries, biconcave shape which gives a large surface area for quick diffusion of oxygen. White Blood cells – Protect the body against infection, change shape to squeeze out of blood vessels to engulf microorganisms. 3. Interpreting disease data (a) Health – State of physical and mental well-being affected by disease, diet, stress and life situations. (b) Diseases: Communicable- spreads between organisms e. g. Ebola (virus) Non-communicable – cannot be spread between organisms e. g Diabetes (c) Diseases can interact: • Defects in the immune system mean someone is more likely to suffer from an infectious disease • Viruses living in cells can trigger cancer • Immune reactions can trigger allergies e. g. Asthma • Severe physical ill health can lead to mental health illnesses (d) Risk factors of diseases: Diet, alcohol, smoking, lack of exercise, carcinogens (e) Casual Mechanisms: The link between some diseases and risk factors e. g Obesity and type 2 diabetes Many diseases are caused by a number of factors. (f) Cancer: Changes in cells leading to uncontrolled growth and division which can form tumours. There are lifestyle and genetic risk factors linked to types of cancer. Tumours: Benign – Abnormal growth of cells contained in one area, they do not invade other parts of the body. Malignant – Invade neighbouring tissues and spread to different parts of the body in the blood to form secondary tumours. Platelets – Fragments of cell collect and cause blood clotting at wounds. 4. Digestion 3 adaptations of the alveoli (i) (a) Digestive system - organ system in which several organs work together to digest and Large, moist surface area (ii) Rich blood supply (iii) Close to blood capillaries = short diffusion distance HT only • After exercise blood flowing through the muscles transports lactic acid to the liver where it is converted back to glucose. • Oxygen debt =. Amount of extra oxygen the body needs after exercise to react with the lactic acid and remove it from cells. Blood vessels: Arteries – Transports blood from heart to organs, thick muscular elastic walls. Veins – Transports blood from organs to heart, thin walls and valves to prevent back flow. Capillaries – Allows transport of substances between cells and Capillary Artery Vein blood, narrow, thin walls. absorb food. (b) Enzymes – Biological catalysts which speed up specific chemical reactions. High temperatures and extreme p. H change the shape of the enzyme, this is called denaturing. This prevents the enzyme from working as the substrate cannot fit in the active site. (c) Lock (active site) and Key (substrate) mechanism

Angle Properties Year 10 – The Knowledge – Science – Biology – Autumn 1 5. Diffusion 4. Digestion (d) Digestion-the breakdown of large insoluble food molecules into small soluble food molecules. Protease – Produced in the stomach, pancreas and small intestine - Proteins Amino Acids Lipase – Produced in the pancreas and small intestine - Lipids Fatty acids + Glycerol Amylase (a carbohydrase) – Produced in the salivary glands and pancreas - Starch Sugar (maltose) (e) Bile: liquid made in the liver and stored in the gall bladder. Increases the rate of breakdown of lipids/fats by lipase. • It is alkaline so neutralises hydrochloric acid in the stomach • Emulsifies fats to form small droplets increasing surface area (f) Food testing: Sugars e. g. glucose – Add Benedict’s reagent, heat in water bath for 2 mins. Positive result = red. Starch – Add Iodine. Positive result = Blue/black (a)Diffusion: Spreading out of particles of any substance in solution or particles of gas, resulting in a net movement from an area of higher concentration to an area of lower concentration e. g. substances may move in and out of cells or across cell membranes - Carbon dioxide and oxygen in gas exchange - Urea from cells into the blood plasma for kidney excretion (b) 3 x Factors affecting the rate of diffusion: i. Difference in concentration gradient ii. Temperature iii. Surface area of the membrane ho (c) Single celled organisms have Large surface area to volume ratio = quicker rate of diffusion (d) Multi cellular organisms smaller surface area to volume ratio, they have adaptations to exchange materials e. g. fish gills, small intestines, roots and leaves: i. Large surface area ii. Thin surface = short diffusion pathway iii. Surface is moist = substances dissolve faster iv. Animals only: Rich blood supply, ventilation. Protein – Add Biuret reagent. Positive result = purple (g) Investigating temperature and p. H – Continuous sampling technique and water bath/ electric heater must be used to control temperature. (h) Metabolism: The sum of all the reactions in a cell or the body Energy transferred by respiration in cells is used by organisms for continual enzyme controlled processes of metabolism that synthesise new molecules including: (e) Calculating surface area: volume ratio = Surface area of object ÷ volume of object • Conversion of glucose to starch, glycogen and cellulose • Formation of lipid molecules from 1 molecule of glycerol and 3 molecules of fatty acid • Glucose and Nitrate ions forming amino acids, which synthesise proteins • Breakdown of excess proteins into Urea for excretion A: Surface area= 6 Volume = 1 SA: volume = 6÷ 1= 6 B: Surface area= 24 Volume = 8 SA: volume = 24÷ 8= 3

Angle Properties Classification Year 11 – The Knowledge – Science – Biology – Autumn 1 Classification - putting living things into groups based on structure and characteristics. 1. Carl Linnaeus - developed the binominal system (organisms have 2 parts to their name) where organisms are grouped into: kingdom phylum class order family genus species (Kids Prefer Candy Over Fried Green Spinach) e. g. Panthera leo = lion Panthera = the ‘genus’ leo = the ‘species’ Evidence for Evolution 1. Fossils - the ‘remains’ of organisms from millions of years ago, which are found in rocks, which show us how much or how little different organisms have changed as life developed on Earth (i. e. how they have evolved). The fossil record – provides evidence for Charles Darwin’s theory of evolution through natural selection Fossils may be formed: • from parts of organisms that have not decayed because moisture (water) or oxygen are absent • when parts of the organism are replaced by minerals as they decay • as preserved traces of organisms, such as footprints, burrows and rootlet traces. Scientists cannot use fossil as evidence for how life began on Earth because: • Many early forms of life were soft-bodied, which means that they have left few traces behind. What traces there were have been mainly destroyed by geological activity. 2. Antibiotic resistance: • bacteria undergo random mutations in their genes, which mean that they are no longer destroyed by an antibiotic • these resistant bacteria are more likely to survive • they are more likely to reproduce quickly through binary fission (asexual reproduction) • bacteria pass on these genes for antibiotic resistance to the next generation • antibiotics are quickly ineffective at destroying resistance bacteria Selective Breeding Selective breeding (artificial selection) - the process by which humans breed plants and animals for particular genetic characteristics. Le Cha Natural selection can be speeded up by humans in selective breeding to increase food production. New ways of classification were developed because: • microscopes improved so scientists knew more about cells • biochemical processes were understood better A species - a group of organisms that can reproduce together to produce offspring that are fertile (can also reproduce). 2. New evidence from genetic studies led to - a three-domain system being developed by Carl Woese where organisms are classified into 3 groups: • archaea e. g. early bacteria living in extreme environments • bacteria e. g. true bacteria such as E. coli • eukaryota e. g. protists, fungi, plants and animals Evolutionary trees - show us how closely organisms are related. They use current classification data for living organisms and fossil data for extinct organisms. Extinction - when there is no remaining member of the species left alive due to: • new predators (slow) • new diseases (slow) • new, more successful competitors (slow) • a single catastrophic event e. g. volcanic eruption. (rapid) Advantages Disadvantages It reduces diseases It can reduce genetic diversity of species It can increase the quality of a product It can result in inbreeding where some breeds are more prone to diseases or defects It can create new varieties of organism that are fitter/stronger It can impact on evolution The steps are: 1. Choose parents with the desired characteristic 2. Breed them together 3. Choose the offspring with the best characteristics 4. Continue over many generations The uses of selective breeding are: • To produce food crops Le Cha • To domesticate animals. The characteristic can be chosen for usefulness or appearance: • Disease resistance in food crops. • Animals which produce more meat or milk. • Domestic dogs with a gentle nature. • Large or unusual flowers.

Angle Properties Genetic engineering Year 11 – The Knowledge – Science – Biology Autumn 1 Genetic engineering - a process which involves modifying the genes of an organism by introducing a gene from another organism to give a desired characteristic. Examples: - Plant crops resistant to diseases or to produce bigger better fruits. - Bacterial cells to produce useful substances such as human insulin - Steps: 1. selection of the desired characteristic 2. isolation of the gene responsible for the characteristic (enzymes are used to cut the gene out) 3. insertion of the gene into another organism called a vector (usually a bacterium) 4. replication of the transgenic organism Homeostasis - homeostasis is the regulation of the internal conditions of a cell or organism to maintain optimum conditions for enzyme to work and all cells to function. Three things controlled by homeostasis are: blood glucose concentration, body temperature, water and ion levels The Endocrine System Endocrine system - composed of glands which secrete chemicals called hormones directly into the bloodstream. The blood carries the hormone to a target organ where it produces an effect. Compared to the nervous system the effects are slower but act for longer. Hormones - chemical messengers that travel around the bloodstream and target an organ, causing the organ to do something. Pituitary gland - a ‘master gland’ which secretes several hormones into the blood, which act on other glands to stimulate other hormones to be released; Controlling Blood Glucose Concentration Advantages It is easier to create high quantities of insulin Insulin is less likely to cause an adverse reaction because it is not taken from a pig. More nutritious food produced Herbicide resistant crop plants increase the yield for farmers. High yield of food can be produced for poorer countries. Disadvantages GM (genetically modified) crops could affect wild flowers and insects. The effects of eating GM crops on human health have not been fully explored. Crossbreeding with and contaminate of wild plants. GM organisms can be expensive Menstrual cycle - 28 day cycle where one egg matures and is released during ovulation Hormone Produced Role Oestrogen Ovaries Makes the lining of the uterus grow again after menstruation Follicle stimulating hormone (FHS) Pituitary gland Causes egg to mature Luteinising hormone (LH) Pituitary gland Stimulates the release of the egg (ovulation) Progesterone Follicle in the ovaries Maintains the lining of the uterus after fertilisation Blood glucose too high: • Pancreas releases insulin • Liver and muscle cells take in and store glucose as glycogen Blood glucose too low: • Pancreas releases glucagon • Liver and muscle cells convert glycogen back to glucose and release it into blood Negative feedback - a way of our body ensuring that any changes are reversed and set back to the correct level e. g. glucagon interacts with insulin in a negative feedback cycle to control blood glucose (sugar) levels Type I diabetes - pancreas fails to produce sufficient insulin leads to: - uncontrolled high blood glucose levels and is - normally treated with insulin injections. Type II diabetes - body cells no longer respond to insulin produced by the pancreas. Treatment: - carbohydrate controlled diet - exercise regime are treatments as - obesity is a risk factor. Controlling The Menstrual Cycle Fertility – how easy it is to become pregnant Contraceptives reduce fertility e. g. oral contraceptive, injection, implant, skin patch, condoms, diaphragms, intrauterine devices, spermicides, abstinence, sterilisation IVF – in vitro fertilisation (egg fertilised by sperm outside of the body) • Mother is given FSH and LH to stimulate the maturation of several eggs. • Eggs fertilised by sperm in the lab • Fertilised eggs develop into embryos • Embryos inserted into mother’s uterus
- Slides: 8