NADH 2 enters the ETC in Step 1







- Slides: 20

NADH 2 enters the ETC in Step #1. FADH 2 does not enter the ETC until Step #3. Steps #1 thru #3: two H+ ions and two electrons are passed from NADH (oxidized back to NAD+) to FMN reducing it to FMNH 2. This passes 2 H+ ions and 2 e- on to Co. Q which is reduced to Co. QH 2. Co. Q can also accept two H+ ions and two electrons from FADH 2. From here, protons stay put, and electrons continue to be transferred.

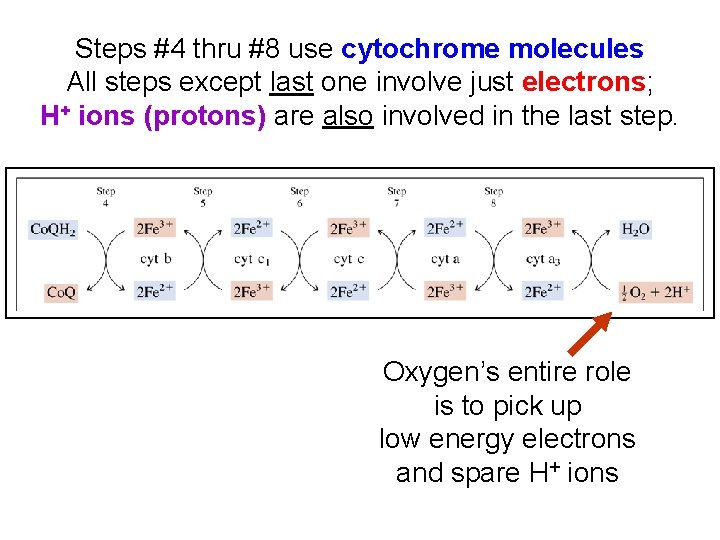
Steps #4 thru #8 use cytochrome molecules All steps except last one involve just electrons; H+ ions (protons) are also involved in the last step. Oxygen’s entire role is to pick up low energy electrons and spare H+ ions

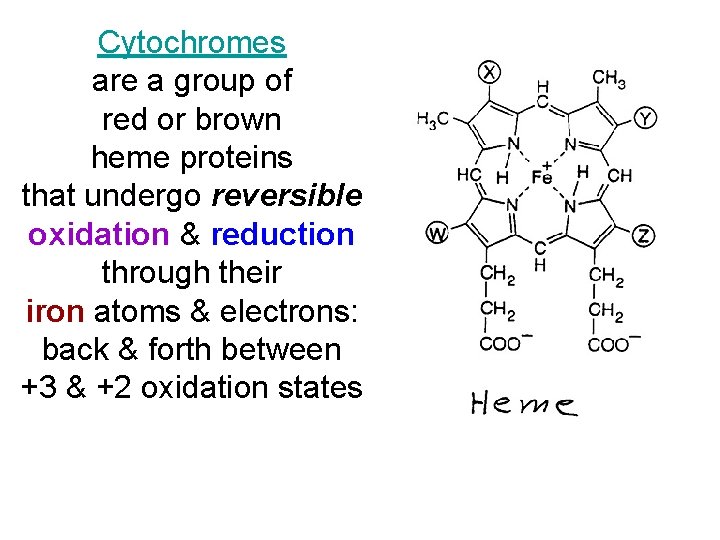
Cytochromes are a group of red or brown heme proteins that undergo reversible oxidation & reduction through their iron atoms & electrons: back & forth between +3 & +2 oxidation states

As the electrons are passed from one cytochrome to another, they lose energy which is indirectly used to make ATP (Wait! We’ll get there) As e- reach the end of the cytochrome chain, they’re picked up & removed by O 2, which reacts with left over hydrogen ions (H+), forming water. This is the water that you sweat, excrete, & breath out.

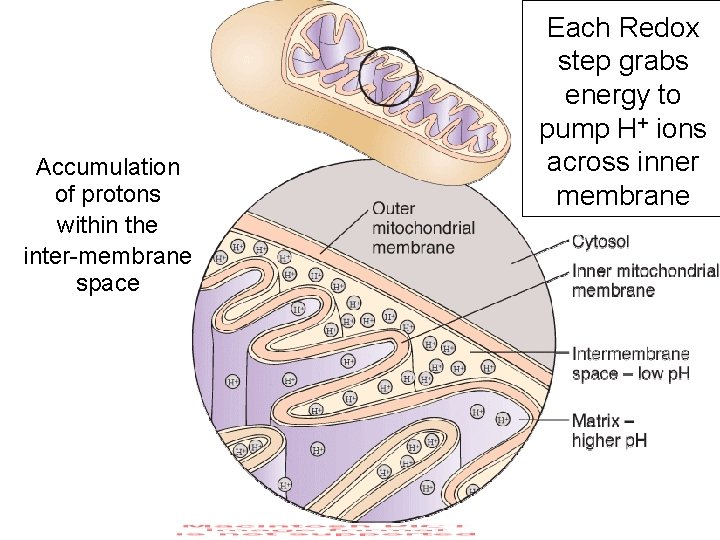
Accumulation of protons within the inter-membrane space Each Redox step grabs energy to pump H+ ions across inner membrane

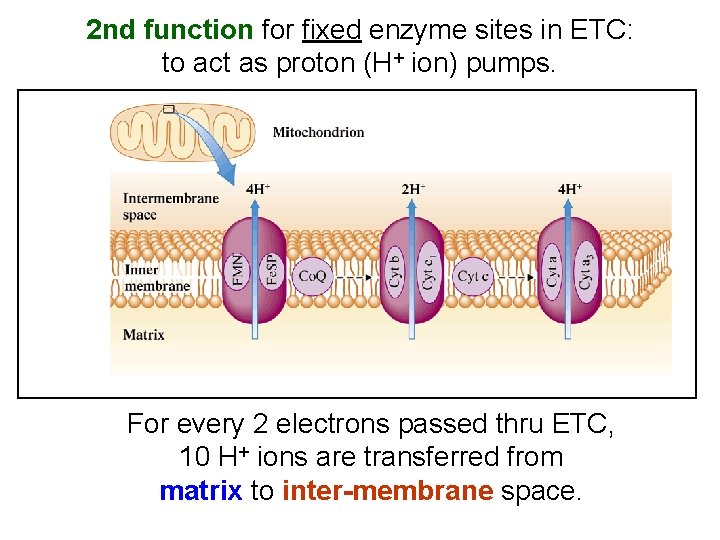
2 nd function for fixed enzyme sites in ETC: to act as proton (H+ ion) pumps. For every 2 electrons passed thru ETC, 10 H+ ions are transferred from matrix to inter-membrane space.

Remember! Diffusion is exergonic Atoms (or ions) are moved in one net direction.

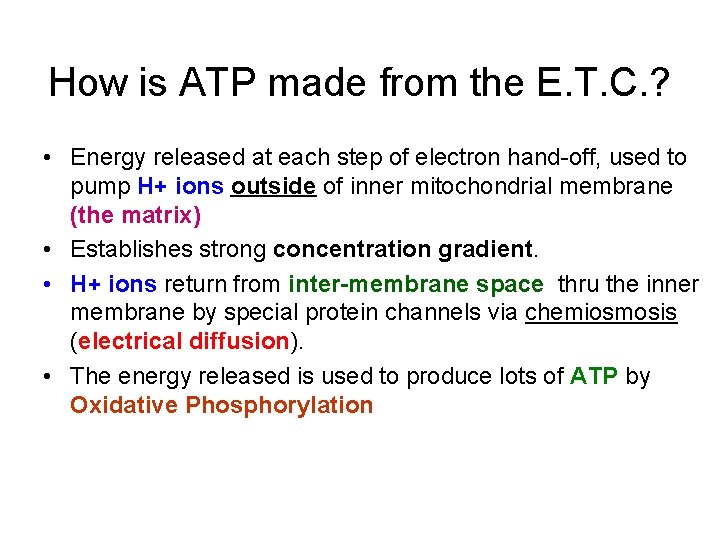
How is ATP made from the E. T. C. ? • Energy released at each step of electron hand-off, used to pump H+ ions outside of inner mitochondrial membrane (the matrix) • Establishes strong concentration gradient. • H+ ions return from inter-membrane space thru the inner membrane by special protein channels via chemiosmosis (electrical diffusion). • The energy released is used to produce lots of ATP by Oxidative Phosphorylation

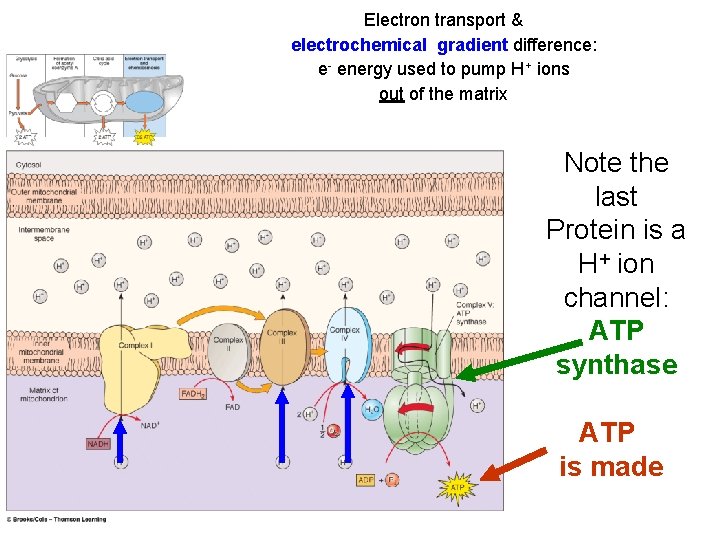
Electron transport & electrochemical gradient difference: e- energy used to pump H+ ions out of the matrix Note the last Protein is a H+ ion channel: ATP synthase ATP is made

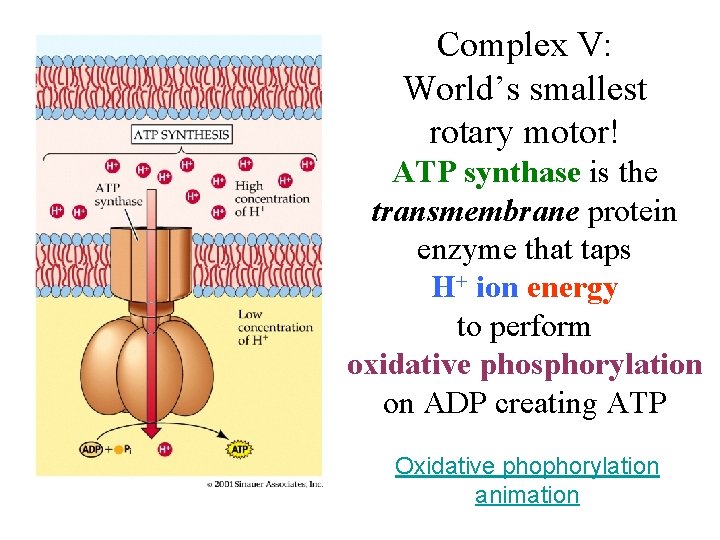
Complex V: World’s smallest rotary motor! ATP synthase is the transmembrane protein enzyme that taps H+ ion energy to perform oxidative phosphorylation on ADP creating ATP Oxidative phophorylation animation

Where’s them mighty-kondreea thingies when I needs ‘em?

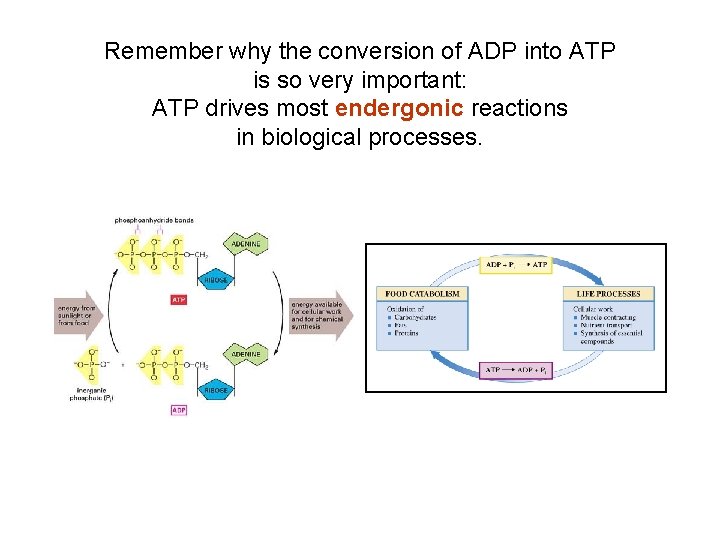
Remember why the conversion of ADP into ATP is so very important: ATP drives most endergonic reactions in biological processes.

• Adenosine triphosphate (ATP) – Holds readily available energy for short periods – Donates energy by means of terminal phosphate group – Strained bonds: repulsive strain = less energy needed to break P-to-P bonds – Most common energy link between • Exergonic and Endergonic reactions

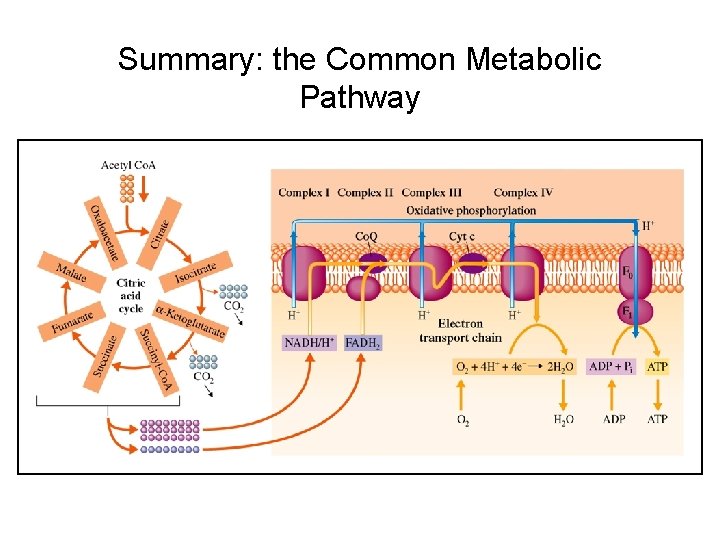
Summary: the Common Metabolic Pathway

TOKYO July 26, 1998 (CNN) -- Japanese police Sunday began investigating whether cyanide was mixed into food served at an annual summer festival in Wakayama, apparently killing four people and sickening dozens of others.

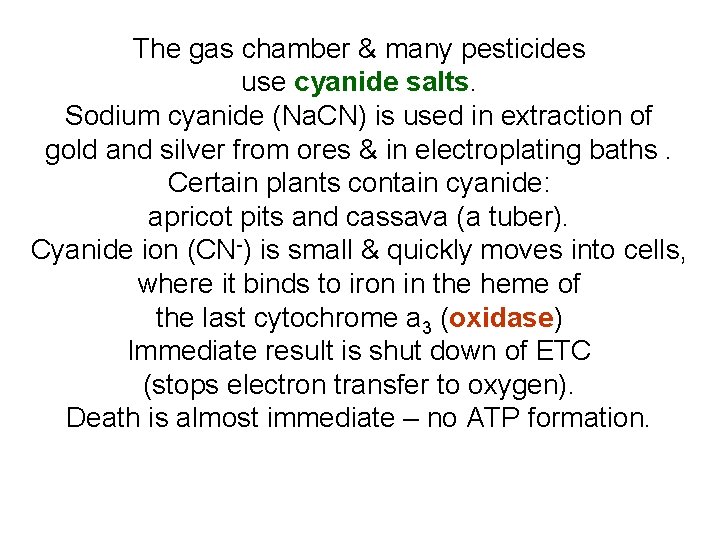
The gas chamber & many pesticides use cyanide salts. Sodium cyanide (Na. CN) is used in extraction of gold and silver from ores & in electroplating baths. Certain plants contain cyanide: apricot pits and cassava (a tuber). Cyanide ion (CN-) is small & quickly moves into cells, where it binds to iron in the heme of the last cytochrome a 3 (oxidase) Immediate result is shut down of ETC (stops electron transfer to oxygen). Death is almost immediate – no ATP formation.

CN- binds to Fe 3+ in oxidase. No transfer of e- to oxygen. Treatment: Nitrites oxidize Fe 2+ in hemoglobin to Fe 3+ & CN- is drawn into bloodstream & converted to SCN- (with thiosulfate)

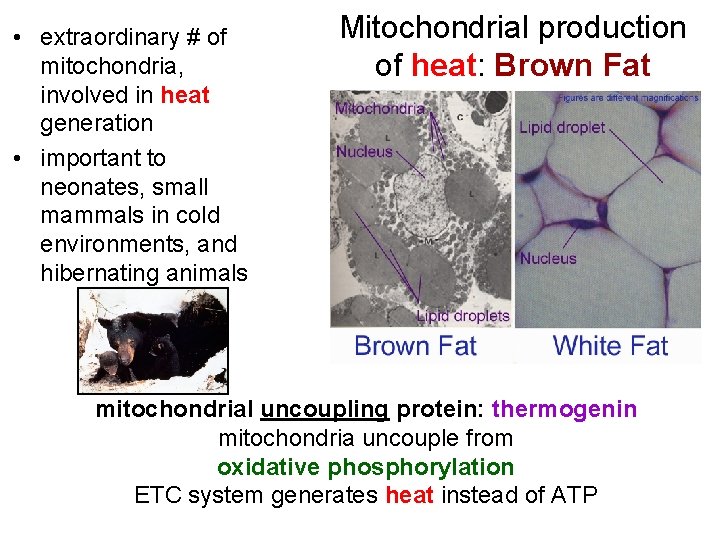
• extraordinary # of mitochondria, involved in heat generation • important to neonates, small mammals in cold environments, and hibernating animals Mitochondrial production of heat: Brown Fat mitochondrial uncoupling protein: thermogenin mitochondria uncouple from oxidative phosphorylation ETC system generates heat instead of ATP

Antioxidants 90% of O 2 used in electron transport chain 10% of O 2 creates reactive oxygen species (ROS) and free radicals like superoxide ions & hydroxyls. Substances like H 2 O 2 and O 2 - and OH are used to kill invading pathogens Afterwards, 95% are decomposed using enzymes: superoxide dismutase and catalase flavonoids & phytochemicals and other antioxidants mop up the remaining free radicals

Flavones Flavonols Antioxidants