Na Oxidation Numbers I lost an electron I

+ (Na ) Oxidation Numbers I lost an electron, I am Positive Dr. Prem D. Sattsangi Christopher L. Byers (programmer) Copyright 2010
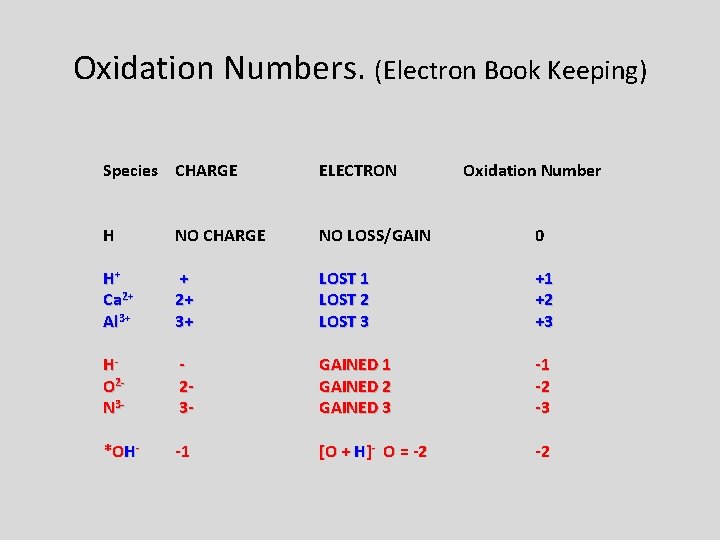
Oxidation Numbers. (Electron Book Keeping) Species CHARGE ELECTRON Oxidation Number H NO CHARGE NO LOSS/GAIN 0 H+ Ca 2+ Al 3+ + 2+ 3+ LOST 1 LOST 2 LOST 3 +1 +2 +3 HO 2 N 3 - 23 - GAINED 1 GAINED 2 GAINED 3 -1 -2 -3 *OH- -1 [O + H]- O = -2 -2

“LEO” the Lion says “GER” Loss of Electron is Oxidation Gain of Electron is Reduction G. E. R. ! REAGENTS (changing) Zn(s) + 2 HCl(aq) O. N. 0 +1 -1 No Change What is the Oxidation No. for? A. Zn(s) = _____ 0 B. Zn in Zn. Cl 2(aq) = _____ +2 C. H 2(g) = _____ 0 D. H in HCl(aq) = _____ +1 E. Cl in HCl(aq) = _____ -1 Use A, B, C, D, E to answer questions. PRODUCTS (forming) Zn. Cl 2(aq) + H 2(g) +2 (-1)2 0 A 1. What is getting oxidized? ______ D 2. What is the oxidizing agent ? ____ 3. What is getting reduced ? _______ D A 4. What is the reducing agent? _____ E 5. Which species has no change? ___
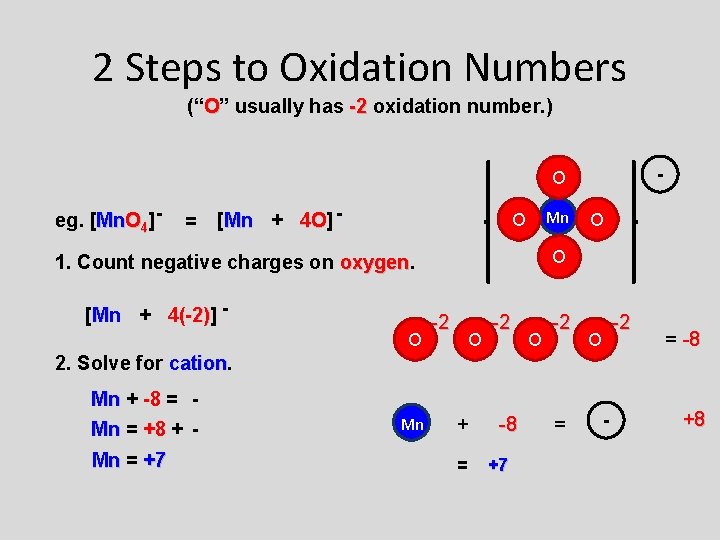
2 Steps to Oxidation Numbers (“O” usually has -2 oxidation number. ) - O eg. [Mn. O 4] - = [Mn + 4 O] 4 O - Mn O O 1. Count negative charges on oxygen [Mn + 4(-2)] 4(-2) O O -2 -2 O = -8 2. Solve for cation Mn + -8 = Mn = +8 + Mn = +7 Mn + = -8 +7 = - +8

Oxidation Numbers What is the Oxidation number of Chlorine in Cl. O 3 -? [Cl. O 3]- = [Cl + 3 O] 3 O - 1. Substitute O with -2. -2 [Cl + 3(-2)] 3(-2) - 2. Solve for cation Cl + -6 = Cl = +6 + Cl = +5
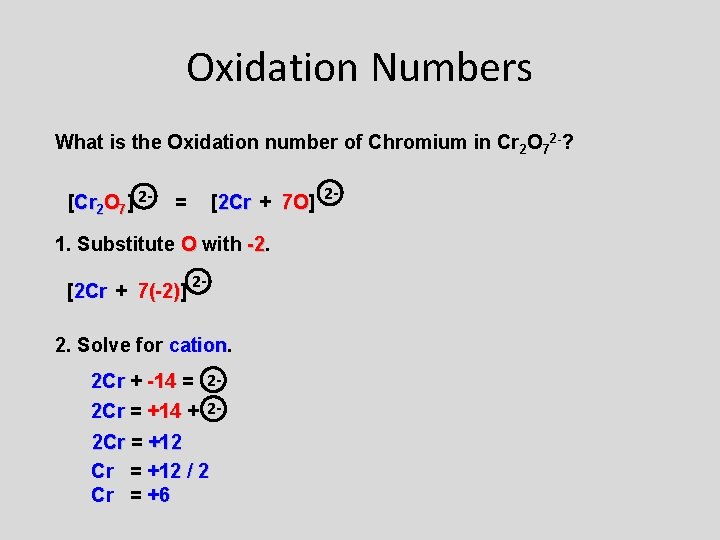
Oxidation Numbers What is the Oxidation number of Chromium in Cr 2 O 72 -? [Cr 2 O 7] 2 - = [2 Cr + 7 O] 7 O 1. Substitute O with -2. -2 [2 Cr + 7(-2)] 7(-2) 2 - 2. Solve for cation 2 Cr + -14 = 22 Cr = +14 + 22 Cr = +12 / 2 Cr = +6 2 -
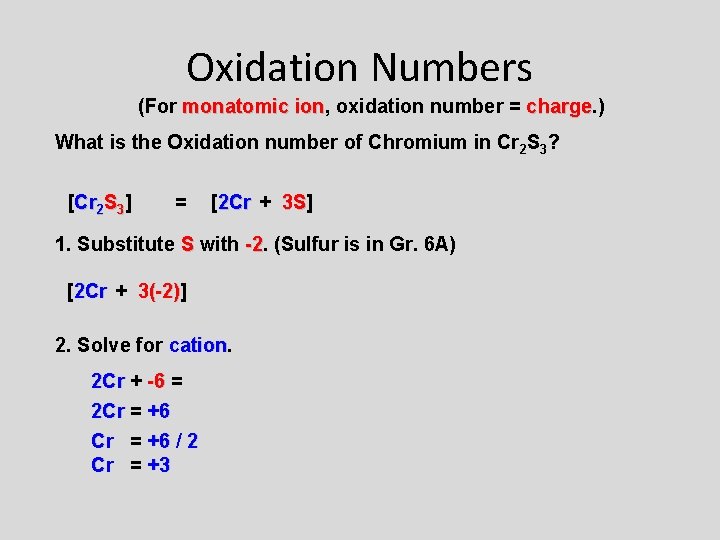
Oxidation Numbers (For monatomic ion, ion oxidation number = charge. ) charge What is the Oxidation number of Chromium in Cr 2 S 3? [Cr 2 S 3] = [2 Cr + 3 S] 3 S 1. Substitute S with -2. -2 (Sulfur is in Gr. 6 A) [2 Cr + 3(-2)] 3(-2) 2. Solve for cation 2 Cr + -6 = 2 Cr = +6 / 2 Cr = +3
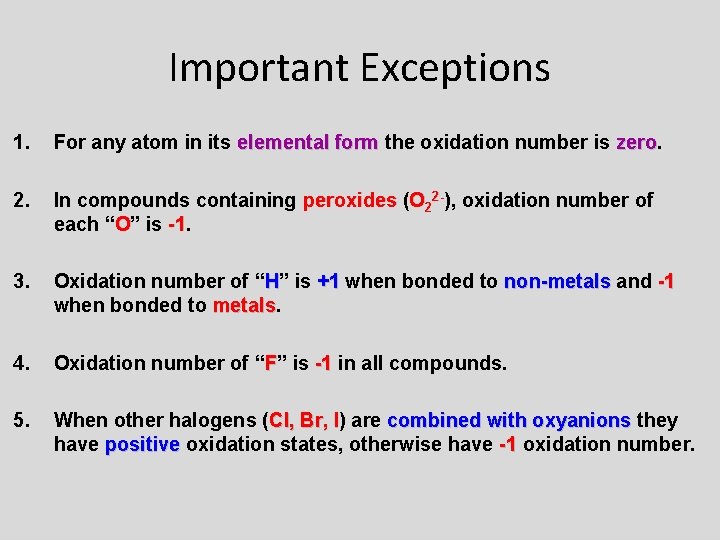
Important Exceptions 1. For any atom in its elemental form the oxidation number is zero 2. In compounds containing peroxides (O 22 -), oxidation number of each “O” is -1. -1 3. Oxidation number of “H” is +1 when bonded to non-metals and -1 when bonded to metals 4. Oxidation number of “F” is -1 in all compounds. 5. When other halogens (Cl, Br, I) I are combined with oxyanions they have positive oxidation states, otherwise have -1 oxidation number.
- Slides: 8