n AMD A Year in Review Key Developments



![EVEREST II: Ranibizumab ± v. PDT in Patients With PCV Study Design[a] BCVA, letters EVEREST II: Ranibizumab ± v. PDT in Patients With PCV Study Design[a] BCVA, letters](https://slidetodoc.com/presentation_image_h/c0fe97e78b560990adb631b67798b715/image-6.jpg)
![ALTAIR: Summary Both T&E regimens improve BCVA and anatomic outcomes[a] • Similar improvement through ALTAIR: Summary Both T&E regimens improve BCVA and anatomic outcomes[a] • Similar improvement through](https://slidetodoc.com/presentation_image_h/c0fe97e78b560990adb631b67798b715/image-15.jpg)
![Emerging Agents: Early Stage Angiopoietins • Key regulator of adult vascular homeostasis[a] • Regulates Emerging Agents: Early Stage Angiopoietins • Key regulator of adult vascular homeostasis[a] • Regulates](https://slidetodoc.com/presentation_image_h/c0fe97e78b560990adb631b67798b715/image-18.jpg)

- Slides: 20

n. AMD – A Year in Review Key Developments From Recent Clinical Trials Moderator Timothy Y. Lai, MD, FRCS, FRCOphth Panelist Annabelle A. Okada, MD, DMSc Professor of Ophthalmology Director, 2010 Retina & Macula Centre Department of Ophthalmology Kyorin University School of Medicine Honorary Clinical Associate Professor Tokyo, Japan Department of Ophthalmology & Visual Sciences The Chinese University of Hong Kowloon, Hong Kong SAR

This program may include a discussion of data that were presented in abstract form. These data should be considered preliminary until published in a peerreviewed journal

Challenges With Current Therapy: Administration High injection frequency leads to high patient volume • Creates backlog of patients • Burden to staff • Burden to patient Financial burden • Treatment not covered by insurance in some countries

Challenges With Current Therapy: Regimens Treat and extend (T&E) • Proactive treatment – Compared to reactive PRN – PRN leads to more visits, more injections § Patients relapse, need to start over from 4 -week injection interval • Reduces patient volume – Patients are treated at every visit • Upper limit of extend period is unclear – Some patients extended to 12, 14, or 16 weeks

Polypoidal Choroidal Vasculopathy 22. 3% to 61. 6% of n. AMD in Asia are PCV[a] Two important trials in PCV • EVEREST II[b] – Intravitreal ranibizumab ± v. PDT – At 12 months compared with ranibizumab monotherapy: § Significantly better VA § Fewer intravitreal ranibizumab injections • PLANET[c] – Aflibercept ± rescue v. PDT for patients with PCV § Study Start Date: May 2014 § Estimated Study Completion Date: July 2017 a. Wong CW, et al. J Clin Med. 2015; 4: 782 -821; b. Koh A, et al. JAMA Ophthalmol. 2017; 135: 1206 -1213; c. Lee WK, et al. ARVO 2017. Oral presentation.
![EVEREST II Ranibizumab v PDT in Patients With PCV Study Designa BCVA letters EVEREST II: Ranibizumab ± v. PDT in Patients With PCV Study Design[a] BCVA, letters](https://slidetodoc.com/presentation_image_h/c0fe97e78b560990adb631b67798b715/image-6.jpg)
EVEREST II: Ranibizumab ± v. PDT in Patients With PCV Study Design[a] BCVA, letters Complete Polyp Regression, % Ranibizumab + v. PDT 8. 3 69. 3 Ranibizumab (single) 5. 1 34. 7 Results at 12 months[b] a. Koh A, et al. AAO 2016. b. Koh A, et al. JAMA Ophthalmol. 2017; 135: 1206 -1213.

PCV: Safety With PDT • PCV first described as a hemorrhagic exudative macular degeneration – Polypoidal lesions by indocyanine green angiography – PDT results in complete regression in 71% to 95% of cases § Stable or improved vision in up to 95% of cases • PDT associated with 2. 2% to 31% hemorrhagic complications Rishi P, et al. Indian J Ophthalmol. 2017; 65: 712 -718.

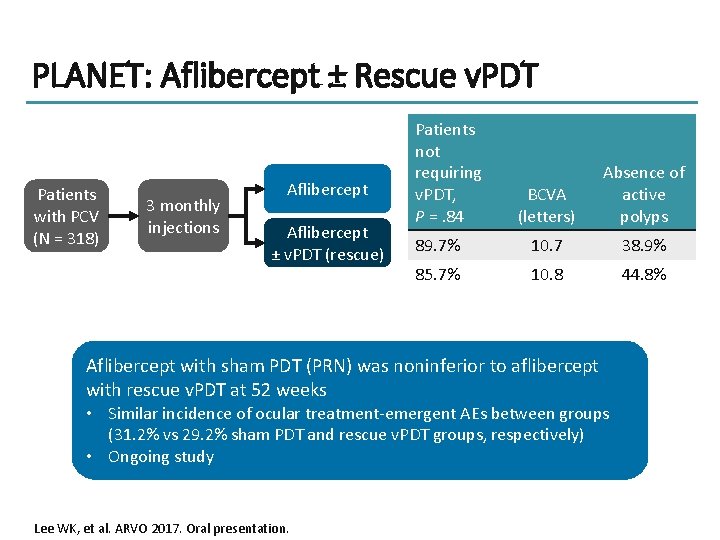
PLANET: Aflibercept ± Rescue v. PDT Patients with PCV (N = 318) 3 monthly injections Aflibercept ± v. PDT (rescue) Patients not requiring v. PDT, P =. 84 BCVA (letters) Absence of active polyps 89. 7% 10. 7 38. 9% 85. 7% 10. 8 44. 8% Aflibercept with sham PDT (PRN) was noninferior to aflibercept with rescue v. PDT at 52 weeks • Similar incidence of ocular treatment-emergent AEs between groups (31. 2% vs 29. 2% sham PDT and rescue v. PDT groups, respectively) • Ongoing study Lee WK, et al. ARVO 2017. Oral presentation.

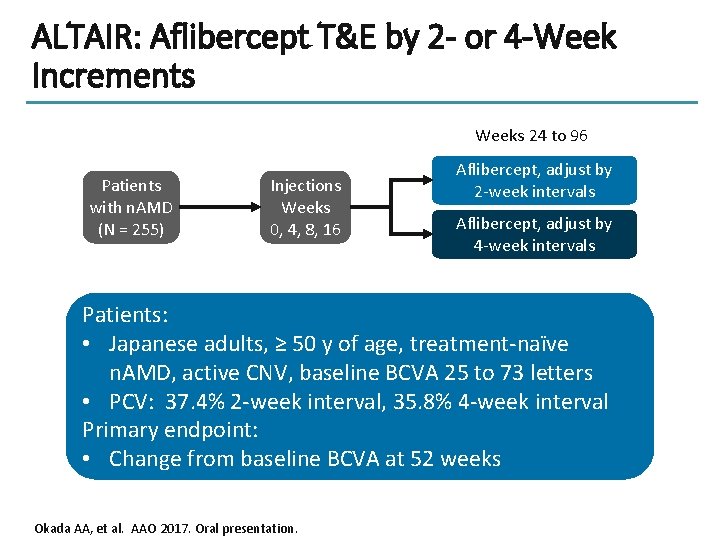
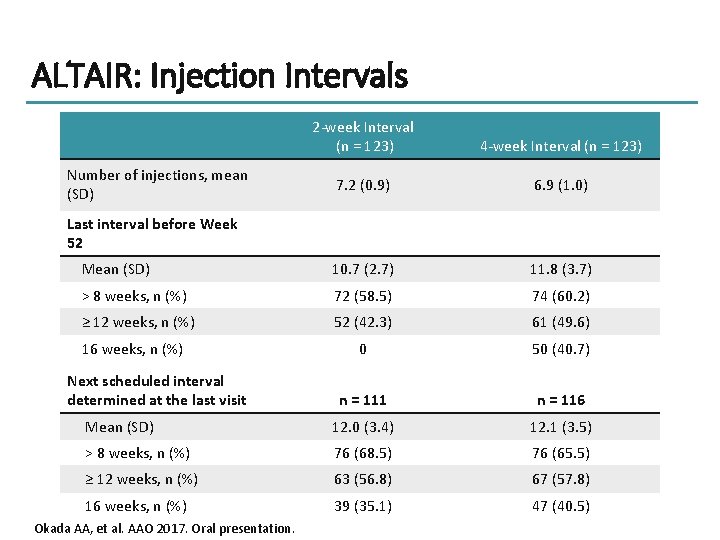
ALTAIR: Aflibercept T&E by 2 - or 4 -Week Increments Weeks 24 to 96 Patients with n. AMD (N = 255) Injections Weeks 0, 4, 8, 16 Aflibercept, adjust by 2 -week intervals Aflibercept, adjust by 4 -week intervals Patients: • Japanese adults, ≥ 50 y of age, treatment-naïve n. AMD, active CNV, baseline BCVA 25 to 73 letters • PCV: 37. 4% 2 -week interval, 35. 8% 4 -week interval Primary endpoint: • Change from baseline BCVA at 52 weeks Okada AA, et al. AAO 2017. Oral presentation.

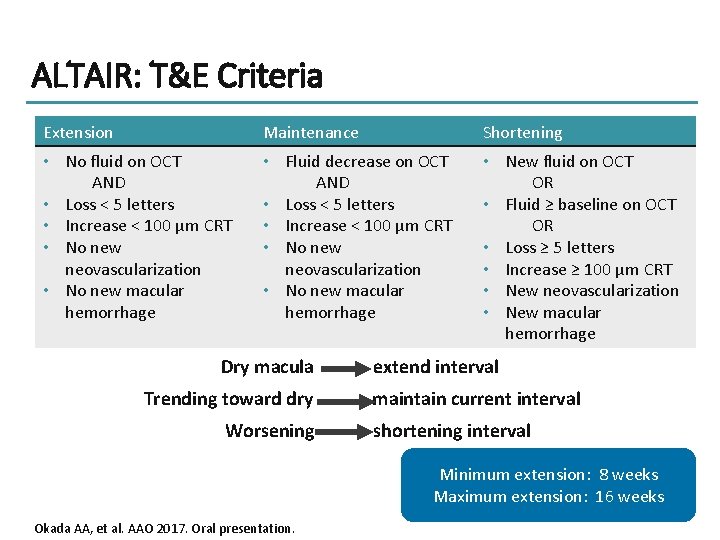
ALTAIR: T&E Criteria Extension Maintenance Shortening • No fluid on OCT AND • Loss < 5 letters • Increase < 100 μm CRT • No new neovascularization • No new macular hemorrhage • Fluid decrease on OCT AND • Loss < 5 letters • Increase < 100 μm CRT • No new neovascularization • No new macular hemorrhage • New fluid on OCT OR • Fluid ≥ baseline on OCT OR • Loss ≥ 5 letters • Increase ≥ 100 μm CRT • New neovascularization • New macular hemorrhage Dry macula Trending toward dry Worsening extend interval maintain current interval shortening interval Minimum extension: 8 weeks Maximum extension: 16 weeks Okada AA, et al. AAO 2017. Oral presentation.

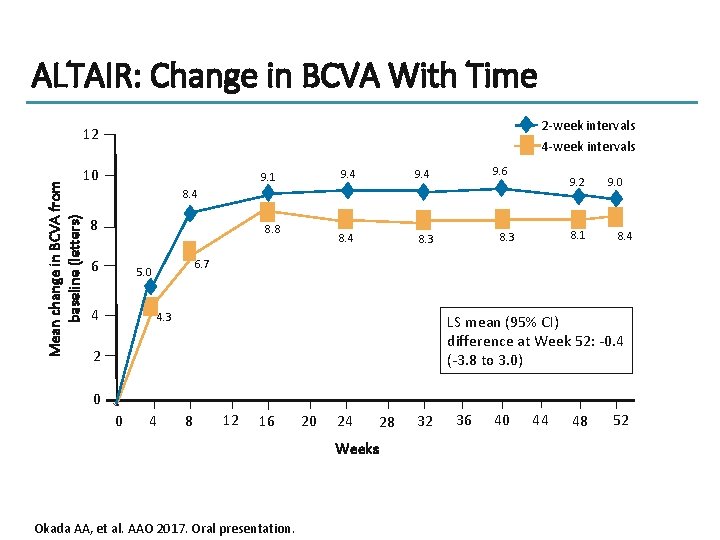
ALTAIR: Change in BCVA With Time 2 -week intervals 4 -week intervals 12 Mean change in BCVA from baseline (letters) 10 9. 6 9. 4 9. 1 9. 2 8. 4 8 8. 8 6 8. 1 8. 3 8. 4 6. 7 5. 0 4 8. 4 9. 0 4. 3 LS mean (95% CI) difference at Week 52: -0. 4 (-3. 8 to 3. 0) 2 0 0 4 8 12 16 20 24 Weeks Okada AA, et al. AAO 2017. Oral presentation. 28 32 36 40 44 48 52

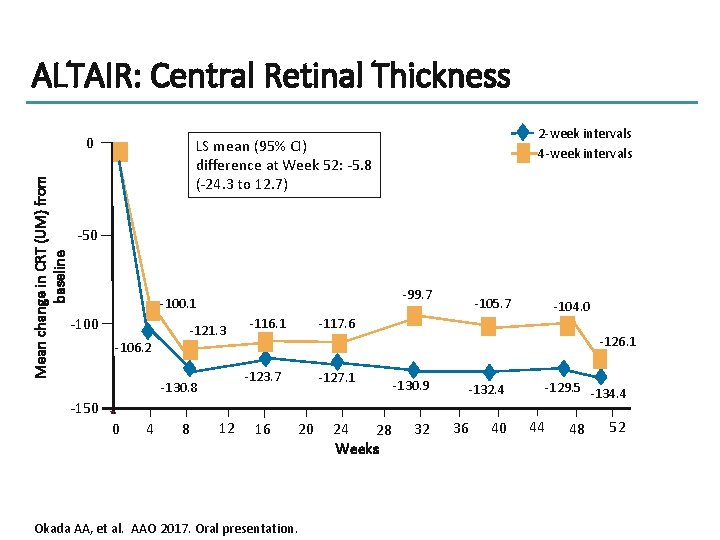
ALTAIR: Central Retinal Thickness Mean change in CRT (UM) from baseline 0 2 -week intervals 4 -week intervals LS mean (95% CI) difference at Week 52: -5. 8 (-24. 3 to 12. 7) -50 -99. 7 -100. 1 -100 -106. 2 -121. 3 -126. 1 -127. 1 -130. 9 -150 0 4 8 12 -104. 0 -117. 6 -116. 1 -123. 7 -130. 8 -105. 7 16 20 Okada AA, et al. AAO 2017. Oral presentation. 24 28 Weeks 32 -132. 4 36 40 -129. 5 -134. 4 44 48 52

ALTAIR: Injection Intervals 2 -week Interval (n = 123) 4 -week Interval (n = 123) 7. 2 (0. 9) 6. 9 (1. 0) Mean (SD) 10. 7 (2. 7) 11. 8 (3. 7) > 8 weeks, n (%) 72 (58. 5) 74 (60. 2) ≥ 12 weeks, n (%) 52 (42. 3) 61 (49. 6) 0 50 (40. 7) n = 111 n = 116 Mean (SD) 12. 0 (3. 4) 12. 1 (3. 5) > 8 weeks, n (%) 76 (68. 5) 76 (65. 5) ≥ 12 weeks, n (%) 63 (56. 8) 67 (57. 8) 16 weeks, n (%) 39 (35. 1) 47 (40. 5) Number of injections, mean (SD) Last interval before Week 52 16 weeks, n (%) Next scheduled interval determined at the last visit Okada AA, et al. AAO 2017. Oral presentation.

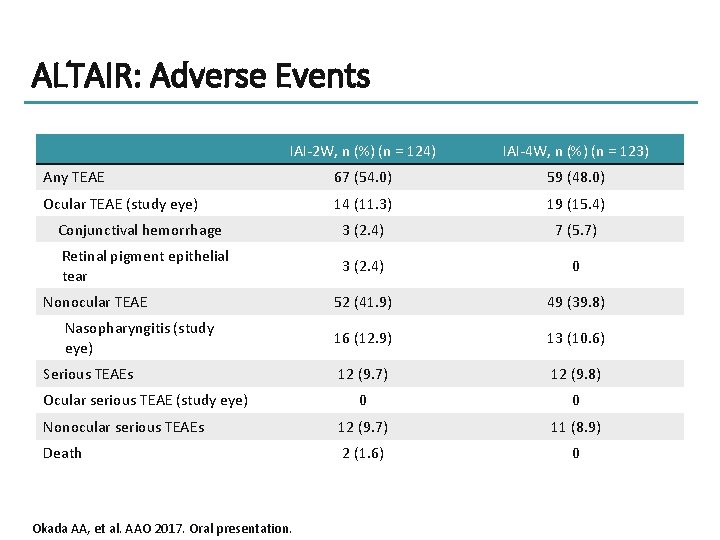
ALTAIR: Adverse Events IAI-2 W, n (%) (n = 124) IAI-4 W, n (%) (n = 123) Any TEAE 67 (54. 0) 59 (48. 0) Ocular TEAE (study eye) 14 (11. 3) 19 (15. 4) Conjunctival hemorrhage 3 (2. 4) 7 (5. 7) Retinal pigment epithelial tear 3 (2. 4) 0 52 (41. 9) 49 (39. 8) 16 (12. 9) 13 (10. 6) 12 (9. 7) 12 (9. 8) 0 0 Nonocular serious TEAEs 12 (9. 7) 11 (8. 9) Death 2 (1. 6) 0 Nonocular TEAE Nasopharyngitis (study eye) Serious TEAEs Ocular serious TEAE (study eye) Okada AA, et al. AAO 2017. Oral presentation.
![ALTAIR Summary Both TE regimens improve BCVA and anatomic outcomesa Similar improvement through ALTAIR: Summary Both T&E regimens improve BCVA and anatomic outcomes[a] • Similar improvement through](https://slidetodoc.com/presentation_image_h/c0fe97e78b560990adb631b67798b715/image-15.jpg)
ALTAIR: Summary Both T&E regimens improve BCVA and anatomic outcomes[a] • Similar improvement through week 52 • 55% had achieved an interval of ≥ 12 weeks – 40% achieved 16 weeks (maximum allowed interval) Findings in line with Asia-Pacific recommendations[b] • Aflibercept dosing intervals can be extended by 4 weeks at a time after 3 -month loading dose • May be extended to 8 - or 12 -week intervals in patients with inactive disease a. Okada AA, et al. AAO 2017. Oral presentation; b. Koh A, et al. Asia Pac J Ophthalmol (Phila). 2017; 6: 296 -302.

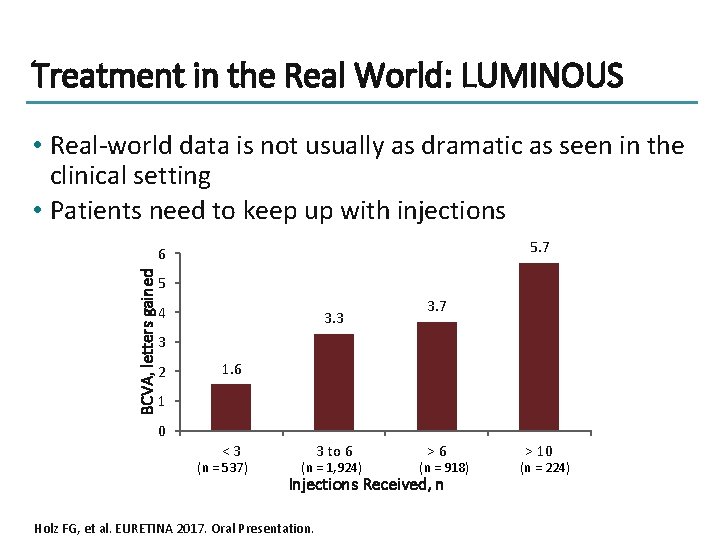
Treatment in the Real World: LUMINOUS • Real-world data is not usually as dramatic as seen in the clinical setting • Patients need to keep up with injections 5. 7 BCVA, letters gained 6 5 4 3. 3 3. 7 3 2 1. 6 1 0 <3 (n = 537) 3 to 6 (n = 1, 924) >6 (n = 918) Injections Received, n Holz FG, et al. EURETINA 2017. Oral Presentation. > 10 (n = 224)

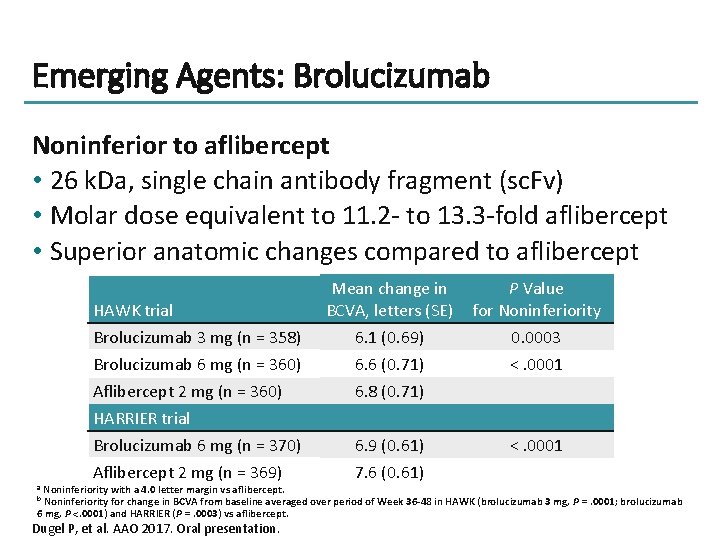
Emerging Agents: Brolucizumab Noninferior to aflibercept • 26 k. Da, single chain antibody fragment (sc. Fv) • Molar dose equivalent to 11. 2 - to 13. 3 -fold aflibercept • Superior anatomic changes compared to aflibercept HAWK trial Brolucizumab 3 mg (n = 358) Brolucizumab 6 mg (n = 360) Aflibercept 2 mg (n = 360) HARRIER trial Brolucizumab 6 mg (n = 370) Aflibercept 2 mg (n = 369) Mean change in BCVA, letters (SE) P Value for Noninferiority 6. 1 (0. 69) 6. 6 (0. 71) 6. 8 (0. 71) 0. 0003 <. 0001 6. 9 (0. 61) 7. 6 (0. 61) <. 0001 Noninferiority with a 4. 0 letter margin vs aflibercept. Noninferiority for change in BCVA from baseline averaged over period of Week 36 -48 in HAWK (brolucizumab 3 mg, P =. 0001; brolucizumab 6 mg, P <. 0001) and HARRIER (P =. 0003) vs aflibercept. a b Dugel P, et al. AAO 2017. Oral presentation.
![Emerging Agents Early Stage Angiopoietins Key regulator of adult vascular homeostasisa Regulates Emerging Agents: Early Stage Angiopoietins • Key regulator of adult vascular homeostasis[a] • Regulates](https://slidetodoc.com/presentation_image_h/c0fe97e78b560990adb631b67798b715/image-18.jpg)
Emerging Agents: Early Stage Angiopoietins • Key regulator of adult vascular homeostasis[a] • Regulates vascular remodeling and maturation[a] • Agents under investigation[b] – RG 7716, ARP-1536, and nesvacumab Other agents and therapies[b] • Anti-VEGF: abicipar pegol, OPT-302, topicals • Anti-PDGF: pegpleranib and rinucumab – Both failed to show consistently improved visual outcomes compared to anti-VEGF monotherapy • Sustained-release anti-VEGF delivery treatments a. Bolinger MT, et al. Int J Mol Sci. 2016; 17: 1498; b. Hussain RM, et al. Expert Opin Emerg Drugs. 2017; 22: 235 -246.

Summary Anti-VEGF improves visual acuity in patients with PCV • EVEREST II and PLANET T&E is a clinically useful approach • Possible to extend treatment intervals faster • Reduces burden on office staff and patients New agents • Brolucizumab • Angiopoietins

Thank you for participating in this activity. To proceed to the online CME test, click on the Earn Credit link on this page.