N 23 Types of Reactions Synthesis Combustion Double
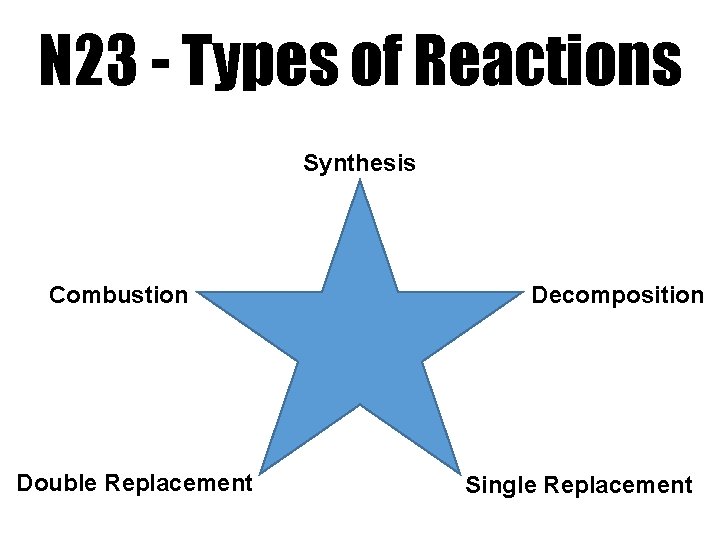
N 23 - Types of Reactions Synthesis Combustion Double Replacement Decomposition Single Replacement

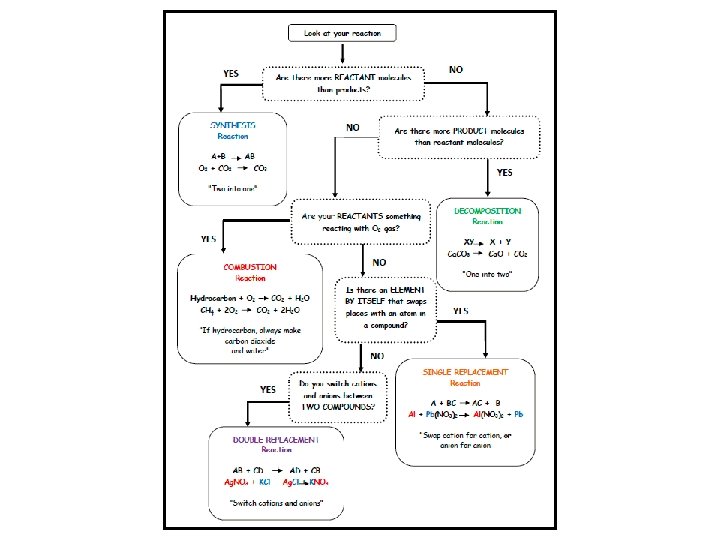
5 Main Categories Helps us predict things about the reactions Know the reactants? You can predict the products Know the products? You can predict the reactants
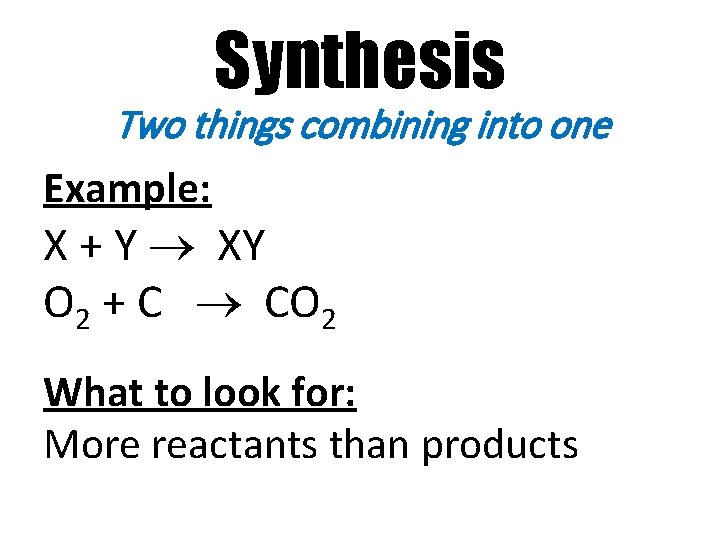
Synthesis Two things combining into one Example: X + Y XY O 2 + C CO 2 What to look for: More reactants than products
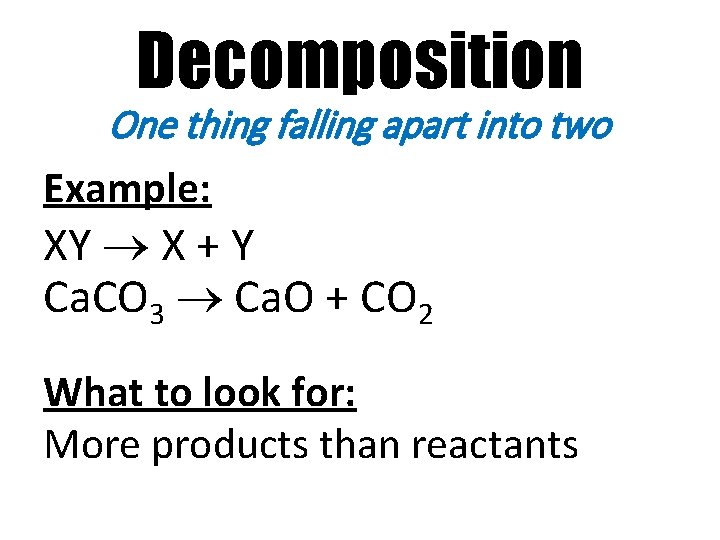
Decomposition One thing falling apart into two Example: XY X + Y Ca. CO 3 Ca. O + CO 2 What to look for: More products than reactants
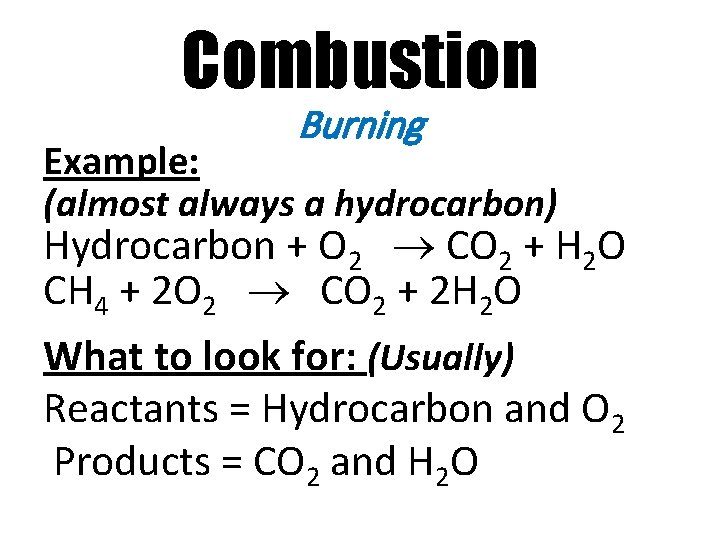
Combustion Burning Example: (almost always a hydrocarbon) Hydrocarbon + O 2 CO 2 + H 2 O CH 4 + 2 O 2 CO 2 + 2 H 2 O What to look for: (Usually) Reactants = Hydrocarbon and O 2 Products = CO 2 and H 2 O
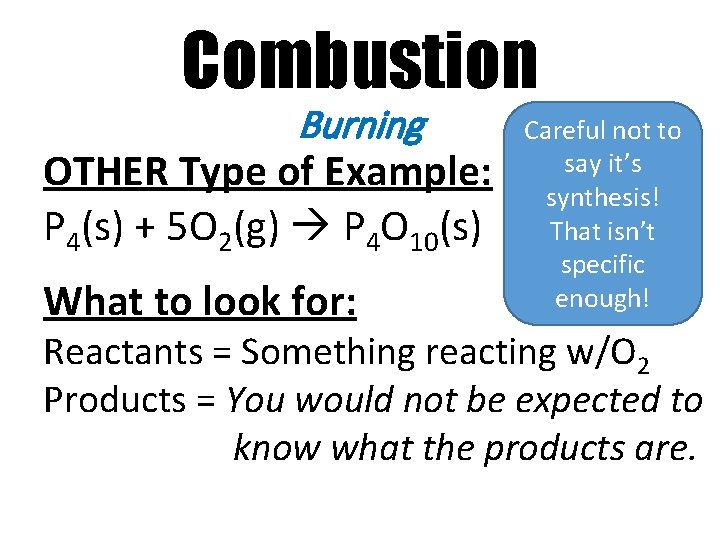
Combustion Burning OTHER Type of Example: P 4(s) + 5 O 2(g) P 4 O 10(s) What to look for: Careful not to say it’s synthesis! That isn’t specific enough! Reactants = Something reacting w/O 2 Products = You would not be expected to know what the products are.
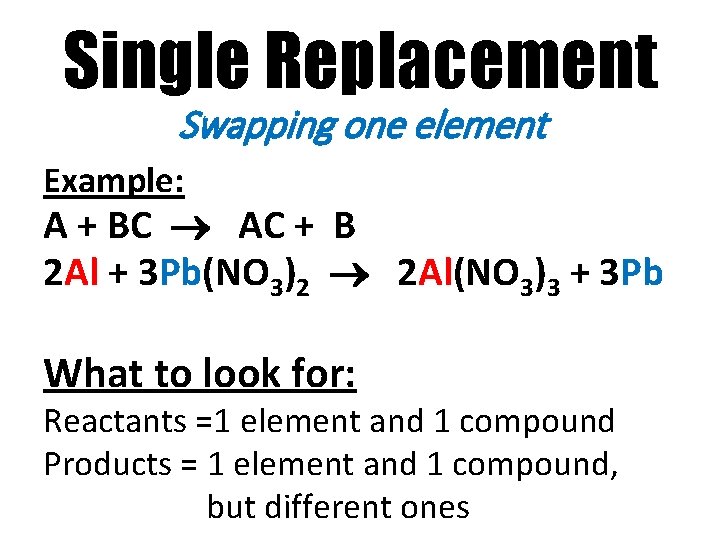
Single Replacement Swapping one element Example: A + BC AC + B 2 Al + 3 Pb(NO 3)2 2 Al(NO 3)3 + 3 Pb What to look for: Reactants =1 element and 1 compound Products = 1 element and 1 compound, but different ones
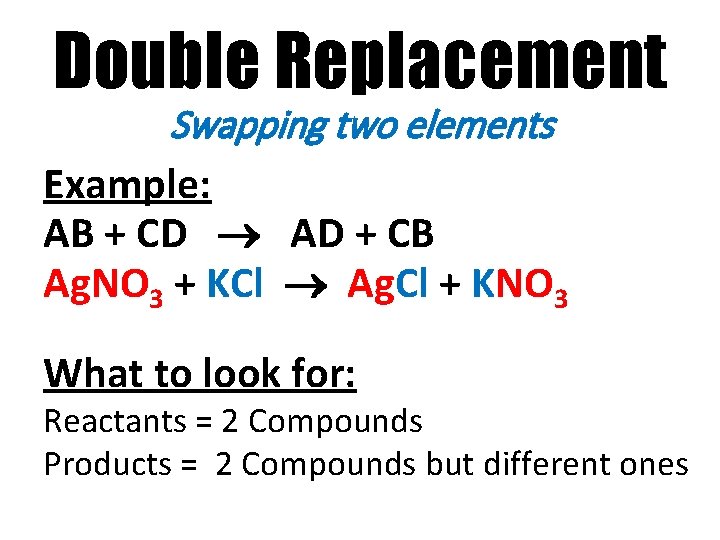
Double Replacement Swapping two elements Example: AB + CD AD + CB Ag. NO 3 + KCl Ag. Cl + KNO 3 What to look for: Reactants = 2 Compounds Products = 2 Compounds but different ones
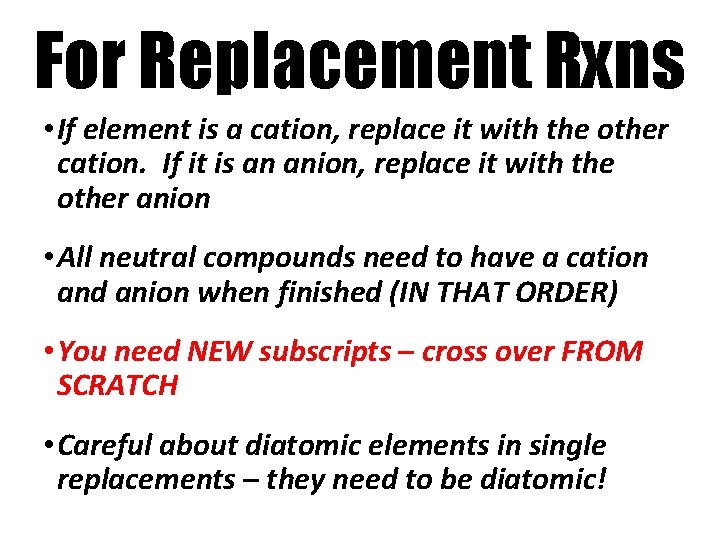
For Replacement Rxns • If element is a cation, replace it with the other cation. If it is an anion, replace it with the other anion • All neutral compounds need to have a cation and anion when finished (IN THAT ORDER) • You need NEW subscripts – cross over FROM SCRATCH • Careful about diatomic elements in single replacements – they need to be diatomic!

You. Tube Link to Presentation: https: //youtu. be/5 q. Jkxya. Odw. Q
- Slides: 11