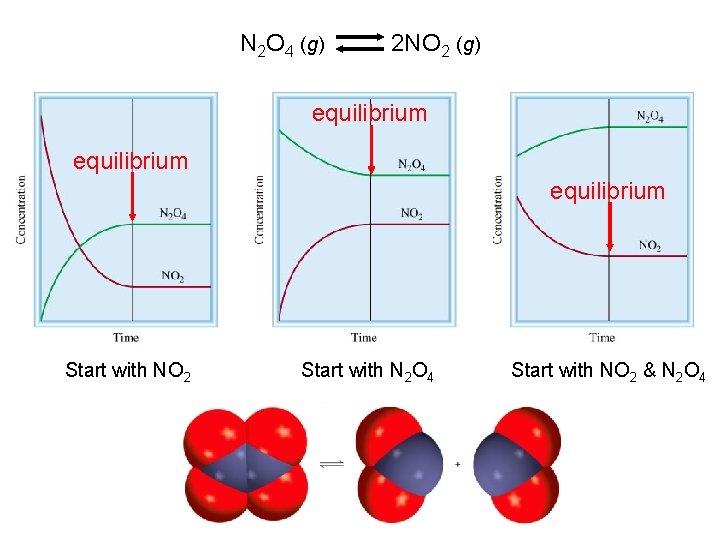
N 2 O 4 g 2 NO 2

![N 2 O 4 (g) 2 NO 2 (g) K = [NO 2]2 [N N 2 O 4 (g) 2 NO 2 (g) K = [NO 2]2 [N](https://slidetodoc.com/presentation_image_h/9a89e3fcba5c9992cb16c621c47967ff/image-6.jpg)
![K = [C]c[D]d a. A + b. B c. C + d. D [A]a[B]b K = [C]c[D]d a. A + b. B c. C + d. D [A]a[B]b](https://slidetodoc.com/presentation_image_h/9a89e3fcba5c9992cb16c621c47967ff/image-8.jpg)


- Slides: 22

N 2 O 4 (g) 2 NO 2 (g) equilibrium Start with NO 2 Start with N 2 O 4 Start with NO 2 & N 2 O 4

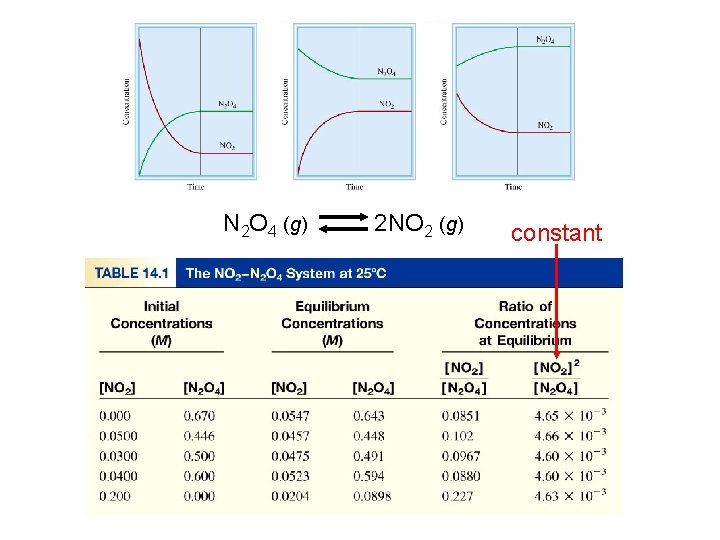
N 2 O 4 (g) 2 NO 2 (g) constant



![N 2 O 4 g 2 NO 2 g K NO 22 N N 2 O 4 (g) 2 NO 2 (g) K = [NO 2]2 [N](https://slidetodoc.com/presentation_image_h/9a89e3fcba5c9992cb16c621c47967ff/image-6.jpg)
N 2 O 4 (g) 2 NO 2 (g) K = [NO 2]2 [N 2 O 4] = 4. 63 x 10 -3 a. A + b. B c. C + d. D K = [C]c[D]d [A]a[B]b Law of Mass Action

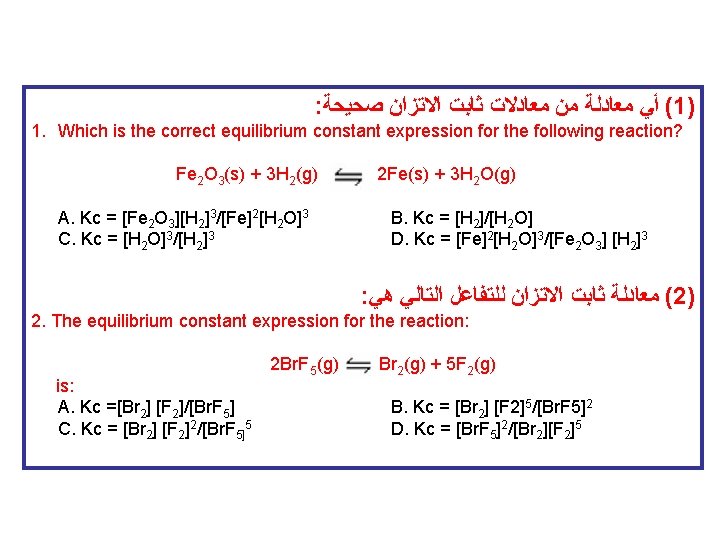
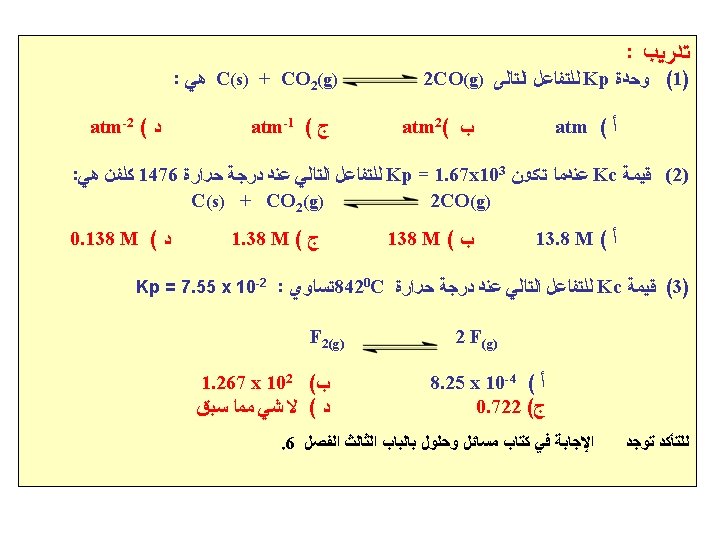
: ﺻﺤﻴﺤﺔ ﺍﻻﺗﺰﺍﻥ ﺛﺎﺑﺖ ﻣﻌﺎﺩﻻﺕ ﻣﻦ ﻣﻌﺎﺩﻟﺔ ﺃﻲ (1) 1. Which is the correct equilibrium constant expression for the following reaction? Fe 2 O 3(s) + 3 H 2(g) 2 Fe(s) + 3 H 2 O(g) A. Kc = [Fe 2 O 3][H 2]3/[Fe]2[H 2 O]3 C. Kc = [H 2 O]3/[H 2]3 B. Kc = [H 2]/[H 2 O] D. Kc = [Fe]2[H 2 O]3/[Fe 2 O 3] [H 2]3 : ﻫﻲ ﺍﻟﺘﺎﻟﻲ ﻟﻠﺘﻔﺎﻋﻞ ﺍﻻﺗﺰﺍﻥ ﺛﺎﺑﺖ ﻣﻌﺎﺩﻟﺔ (2) 2. The equilibrium constant expression for the reaction: 2 Br. F 5(g) Br 2(g) + 5 F 2(g) is: A. Kc =[Br 2] [F 2]/[Br. F 5] B. Kc = [Br 2] [F 2]5/[Br. F 5]2 C. Kc = [Br 2] [F 2]2/[Br. F 5]5 D. Kc = [Br. F 5]2/[Br 2][F 2]5
![K CcDd a A b B c C d D AaBb K = [C]c[D]d a. A + b. B c. C + d. D [A]a[B]b](https://slidetodoc.com/presentation_image_h/9a89e3fcba5c9992cb16c621c47967ff/image-8.jpg)
K = [C]c[D]d a. A + b. B c. C + d. D [A]a[B]b Equilibrium Will K >> 1 Lie to the right Favor products K << 1 Lie to the left Favor reactants

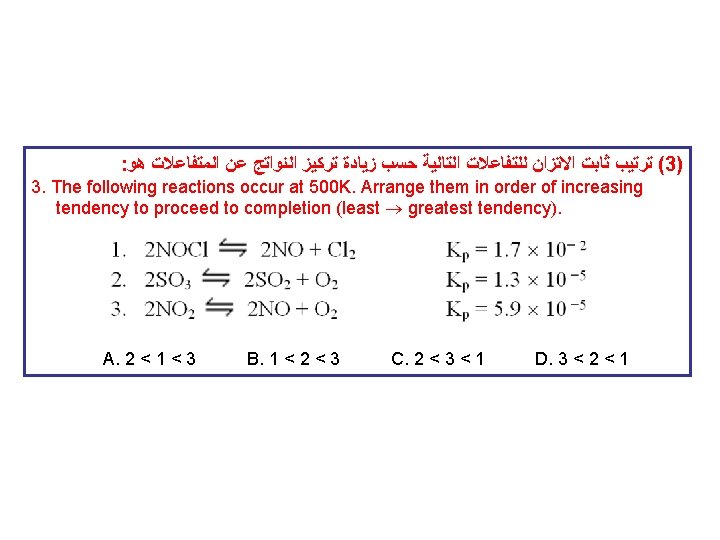
: ﻫﻮ ﺍﻟﻤﺘﻔﺎﻋﻼﺕ ﻋﻦ ﺍﻟﻨﻮﺍﺗﺞ ﺗﺮﻛﻴﺰ ﺯﻳﺎﺩﺓ ﺣﺴﺐ ﺍﻟﺘﺎﻟﻴﺔ ﻟﻠﺘﻔﺎﻋﻼﺕ ﺍﻻﺗﺰﺍﻥ ﺛﺎﺑﺖ ﺗﺮﺗﻴﺐ (3) 3. The following reactions occur at 500 K. Arrange them in order of increasing tendency to proceed to completion (least greatest tendency). A. 2 < 1 < 3 B. 1 < 2 < 3 C. 2 < 3 < 1 D. 3 < 2 < 1

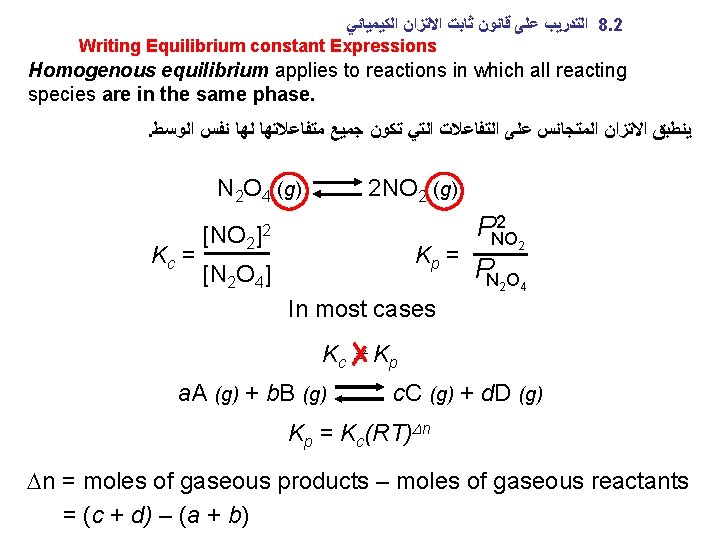
ﺍﻟﻜﻴﻤﻴﺎﺋﻲ ﺍﻻﺗﺰﺍﻥ ﺛﺎﺑﺖ ﻗﺎﻧﻮﻥ ﻋﻠﻰ ﺍﻟﺘﺪﺭﻳﺐ 8. 2 Writing Equilibrium constant Expressions Homogenous equilibrium applies to reactions in which all reacting species are in the same phase. . ﺍﻟﻮﺳﻂ ﻧﻔﺲ ﻟﻬﺎ ﻣﺘﻔﺎﻋﻼﺗﻬﺎ ﺟﻤﻴﻊ ﺗﻜﻮﻥ ﺍﻟﺘﻲ ﺍﻟﺘﻔﺎﻋﻼﺕ ﻋﻠﻰ ﺍﻟﻤﺘﺠﺎﻧﺲ ﺍﻻﺗﺰﺍﻥ ﻳﻨﻄﺒﻖ N 2 O 4 (g) 2 NO 2 (g) Kc = [NO 2 ]2 Kp = [N 2 O 4] 2 PNO 2 PN 2 O 4 In most cases Kc K p a. A (g) + b. B (g) c. C (g) + d. D (g) Kp = Kc(RT)Dn Dn = moles of gaseous products – moles of gaseous reactants = (c + d) – (a + b)

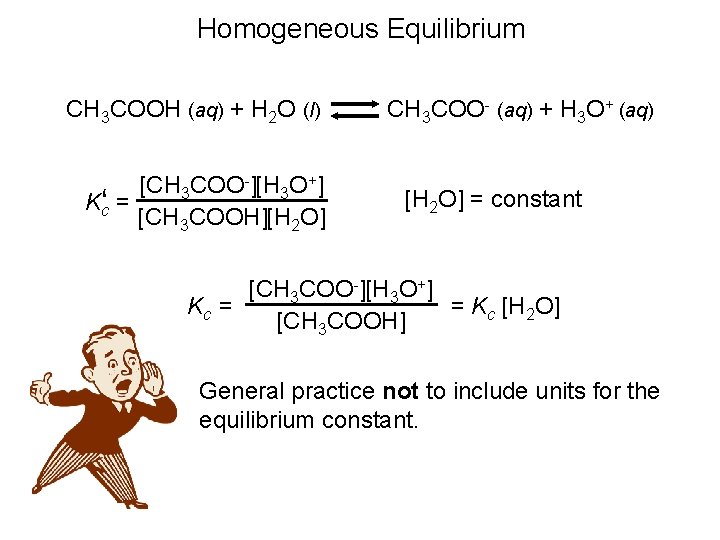
Homogeneous Equilibrium CH 3 COOH (aq) + H 2 O (l) CH 3 COO- (aq) + H 3 O+ (aq) [CH 3 COO-][H 3 O+] Kc‘ = [CH 3 COOH][H 2 O] = constant [CH 3 COO-][H 3 O+] = Kc [H 2 O] Kc = [CH 3 COOH] General practice not to include units for the equilibrium constant.

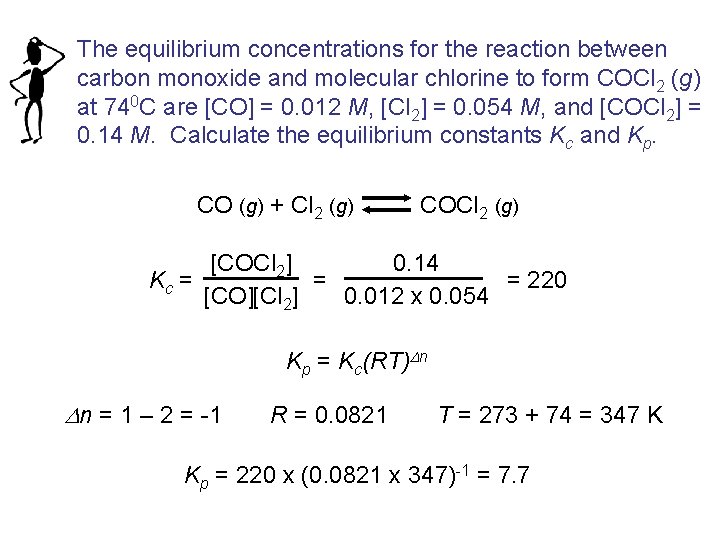
The equilibrium concentrations for the reaction between carbon monoxide and molecular chlorine to form COCl 2 (g) at 740 C are [CO] = 0. 012 M, [Cl 2] = 0. 054 M, and [COCl 2] = 0. 14 M. Calculate the equilibrium constants Kc and Kp. CO (g) + Cl 2 (g) COCl 2 (g) [COCl 2] 0. 14 = = 220 Kc = [CO][Cl 2] 0. 012 x 0. 054 Kp = Kc(RT)Dn Dn = 1 – 2 = -1 R = 0. 0821 T = 273 + 74 = 347 K Kp = 220 x (0. 0821 x 347)-1 = 7. 7

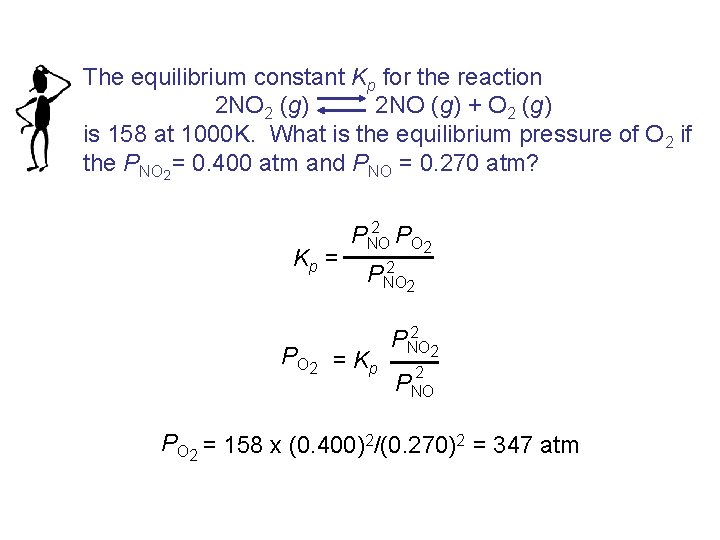
The equilibrium constant Kp for the reaction 2 NO 2 (g) 2 NO (g) + O 2 (g) is 158 at 1000 K. What is the equilibrium pressure of O 2 if the PNO 2 = 0. 400 atm and PNO = 0. 270 atm? Kp = 2 PNO PO 2 2 PNO 2 PO 2 = Kp 2 PNO 2 2 PNO PO 2 = 158 x (0. 400)2/(0. 270)2 = 347 atm

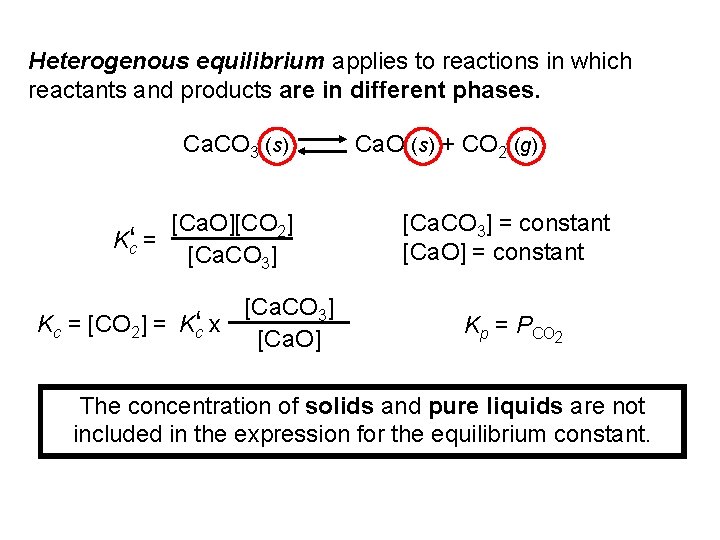
Heterogenous equilibrium applies to reactions in which reactants and products are in different phases. Ca. CO 3 (s) Ca. O (s) + CO 2 (g) [Ca. O][CO 2] Kc‘ = [Ca. CO 3] Kc = [CO 2] = Kc‘ x [Ca. O] [Ca. CO 3] = constant [Ca. O] = constant Kp = PCO 2 The concentration of solids and pure liquids are not included in the expression for the equilibrium constant.

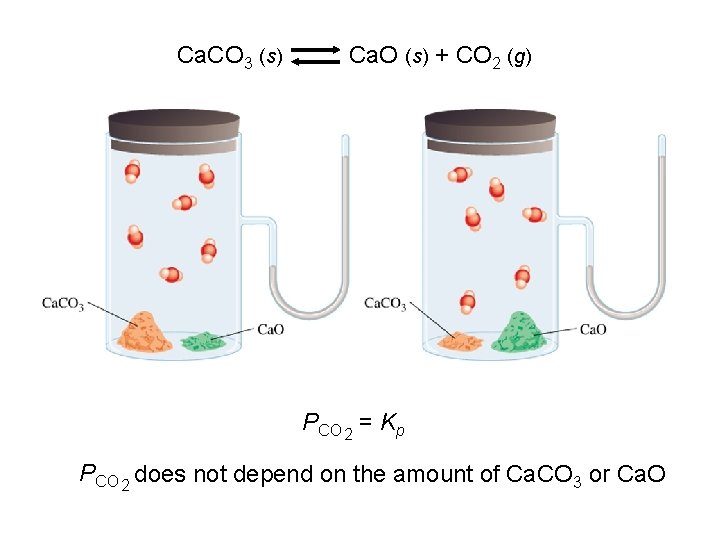
Ca. CO 3 (s) Ca. O (s) + CO 2 (g) PCO 2 = Kp PCO 2 does not depend on the amount of Ca. CO 3 or Ca. O

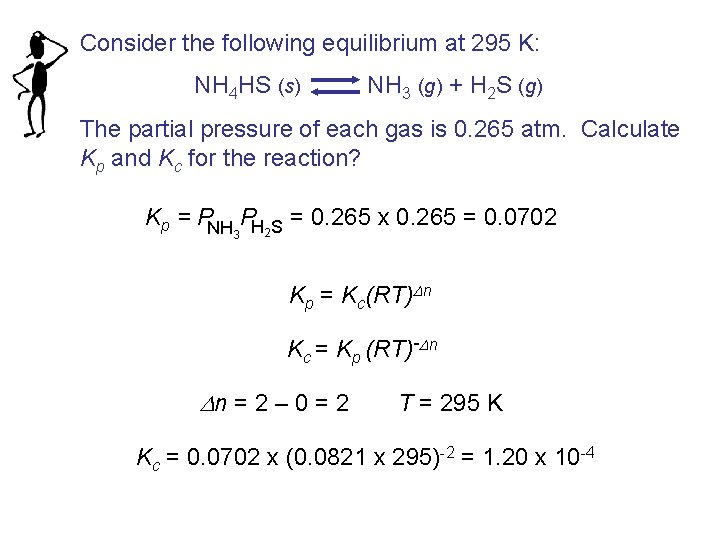
Consider the following equilibrium at 295 K: NH 4 HS (s) NH 3 (g) + H 2 S (g) The partial pressure of each gas is 0. 265 atm. Calculate Kp and Kc for the reaction? Kp = PNH PH S = 0. 265 x 0. 265 = 0. 0702 3 2 Kp = Kc(RT)Dn Kc = Kp (RT)-Dn Dn = 2 – 0 = 2 T = 295 K Kc = 0. 0702 x (0. 0821 x 295)-2 = 1. 20 x 10 -4


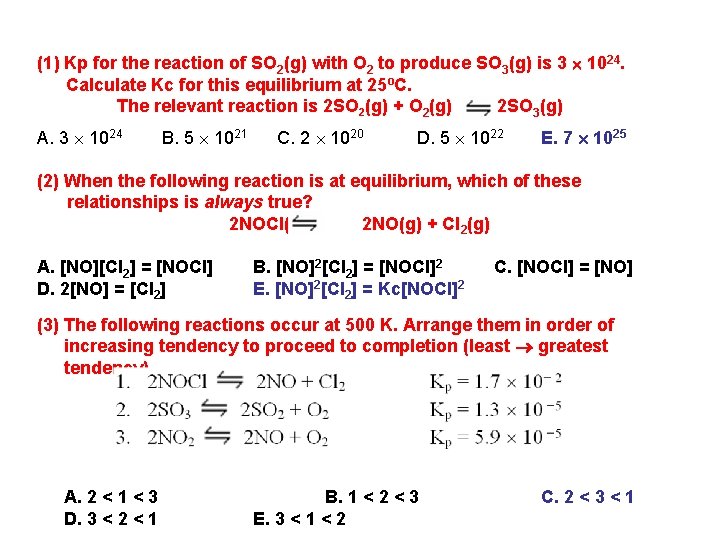
(1) Kp for the reaction of SO 2(g) with O 2 to produce SO 3(g) is 3 1024. Calculate Kc for this equilibrium at 25ºC. The relevant reaction is 2 SO 2(g) + O 2(g) 2 SO 3(g) A. 3 1024 B. 5 1021 C. 2 1020 D. 5 1022 E. 7 1025 (2) When the following reaction is at equilibrium, which of these relationships is always true? 2 NOCl(g) 2 NO(g) + Cl 2(g) A. [NO][Cl 2] = [NOCl] D. 2[NO] = [Cl 2] B. [NO]2[Cl 2] = [NOCl]2 C. [NOCl] = [NO] E. [NO]2[Cl 2] = Kc[NOCl]2 (3) The following reactions occur at 500 K. Arrange them in order of increasing tendency to proceed to completion (least greatest tendency). A. 2 < 1 < 3 D. 3 < 2 < 1 B. 1 < 2 < 3 E. 3 < 1 < 2 C. 2 < 3 < 1

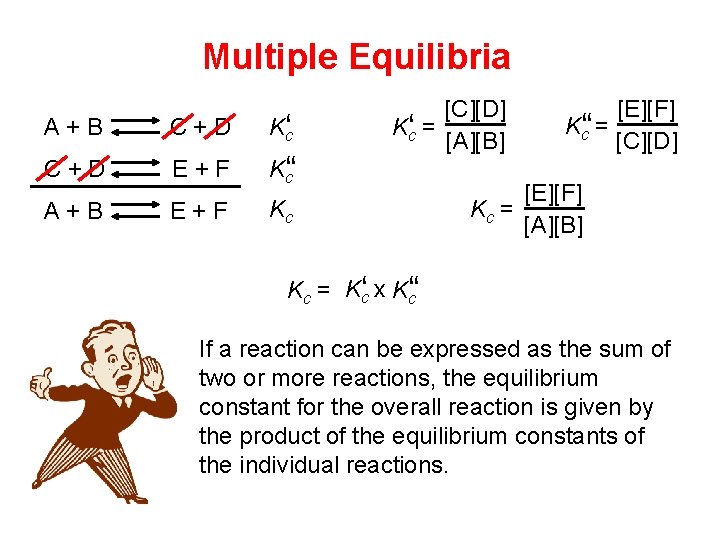
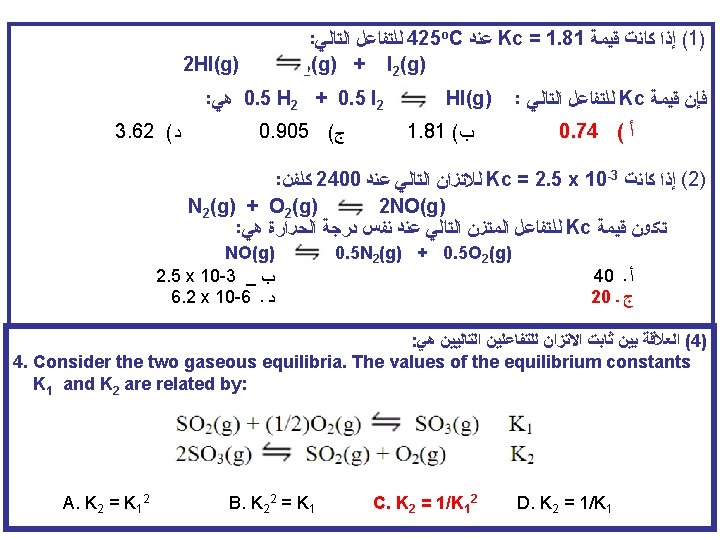
Multiple Equilibria A + B C + D Kc‘ C + D E + F K‘c‘ A + B E + F Kc [C][D] Kc‘ = [A][B] [E][F] Kc‘‘ = [C][D] [E][F] Kc = [A][B] Kc = Kc‘ x K‘c‘ If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions.



Writing Equilibrium Constant Expressions 1. The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium constant is a dimensionless quantity. 4. In quoting a value for the equilibrium constant, you must specify the balanced equation and the temperature. 5. If a reaction can be expressed as a sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions.