Myotonic dystrophy Cardiac phenotype risk stratification and management








- Slides: 34

Myotonic dystrophy: Cardiac phenotype, risk stratification and management Mark Hamilton, (formerly) Clinical Research Fellow West of Scotland Clinical Genetics Service markhamilton 1@nhs. net

Scottish management guidelines for myotonic dystrophy www. smn. scot. nhs. uk Ayrshire Victor Chong, Crosshouse Hospital Borders Paul Neary, Borders General Hospital Dumfries & Galloway Graeme Tait, Dumfries and Galloway Royal Infirmary Fife Mark Francis, Victoria Hospital Forth Valley Catherine Labinjoh, Forth Valley Royal Hospital Greater Glasgow & Clyde David Murdoch, Caroline Coats, Iain Findlay, Queen Elizabeth University Hospital Highland Stephen Cross, Raigmore Hospital Lanarkshire Andrew Docherty, Wishaw General Hospital Brian O’Rourke Hairmyres Hospital Lothian Martin Denvir, Royal Infirmary of Edinburgh Alan Japp, Royal Infirmary of Edinburgh Tayside Anna Maria Choy, Ninewells Hospital

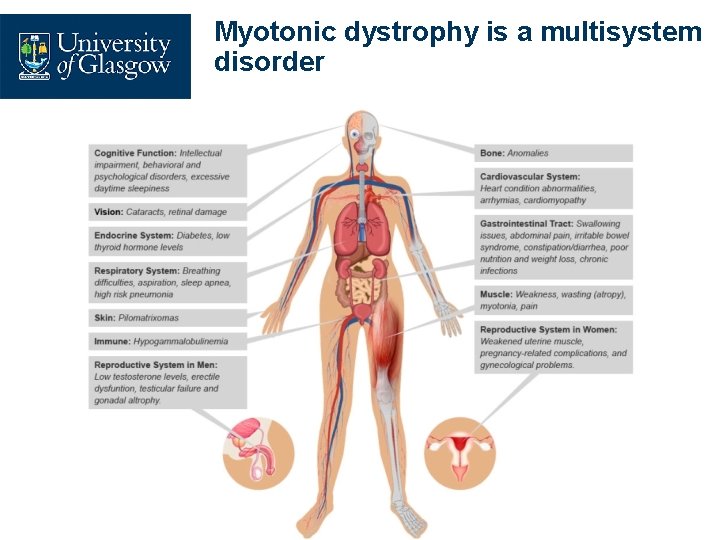
Overview • Molecular mechanisms in myotonic dystrophy • Not just a muscle disease! • Cardiac phenotype • Bradyarrhythmia • Cardiomyopathy • Ventricular dysrhythmias • Potential care standards

Myotonic dystrophy type 1: Quick facts • Commonest inherited muscle disease in adults • 1 in 8, 000 • Complex, multi-system condition • “Probably the most variable disorder known in medicine” Pathogenic n. 50 to > 1, 000 Premutation n. 38 to 49 General population n. 5 to 37 DMPK CTG(n)

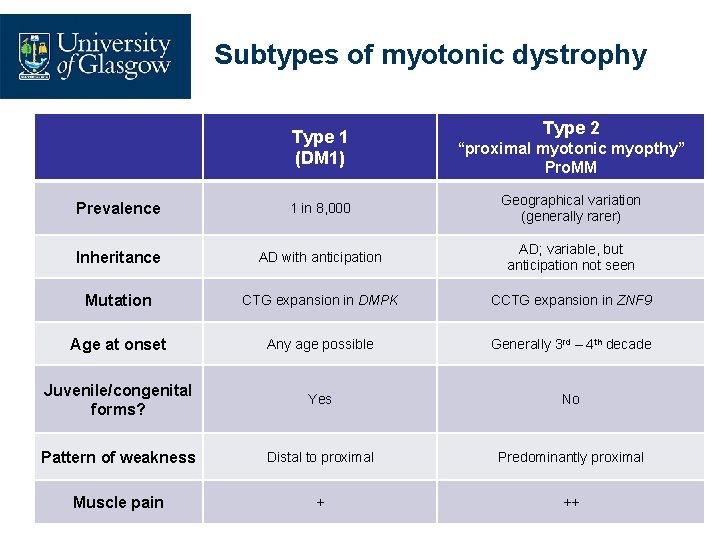
Subtypes of myotonic dystrophy Type 1 (DM 1) Type 2 “proximal myotonic myopthy” Pro. MM Prevalence 1 in 8, 000 Geographical variation (generally rarer) Inheritance AD with anticipation AD; variable, but anticipation not seen Mutation CTG expansion in DMPK CCTG expansion in ZNF 9 Age at onset Any age possible Generally 3 rd – 4 th decade Juvenile/congenital forms? Yes No Pattern of weakness Distal to proximal Predominantly proximal Muscle pain + ++

What goes wrong in myotonic dystrophy?

What goes wrong in myotonic dystrophy? DNA m. RNA

What goes wrong in myotonic dystrophy? DNA m. RNA

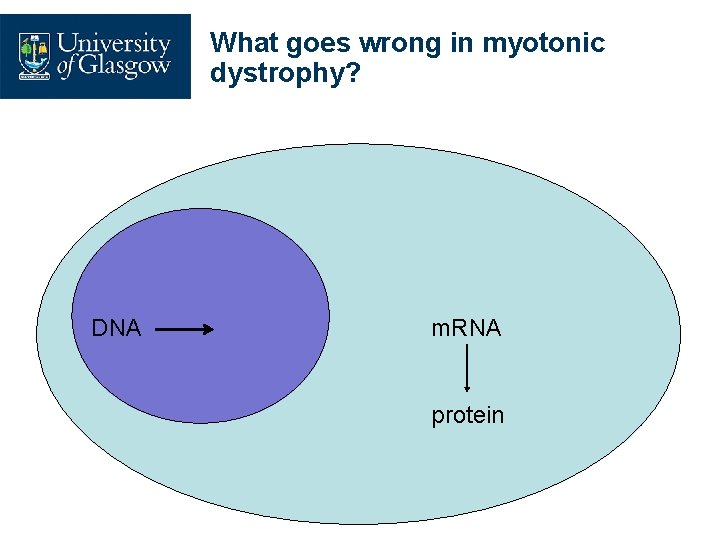
What goes wrong in myotonic dystrophy? DNA m. RNA protein

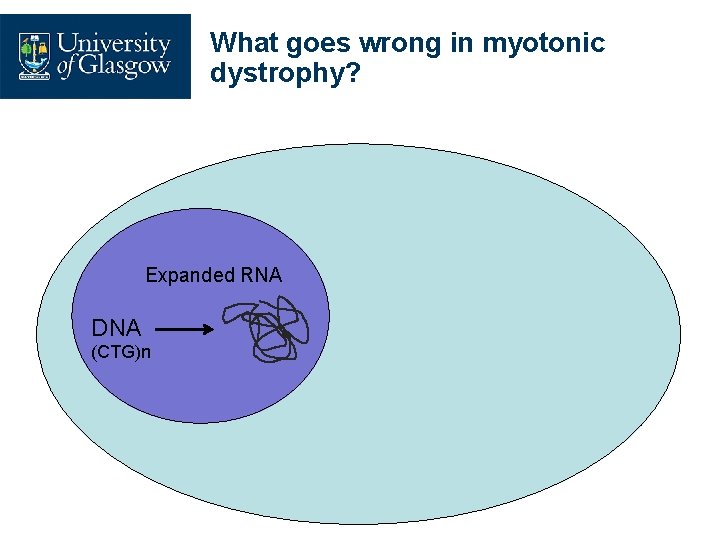
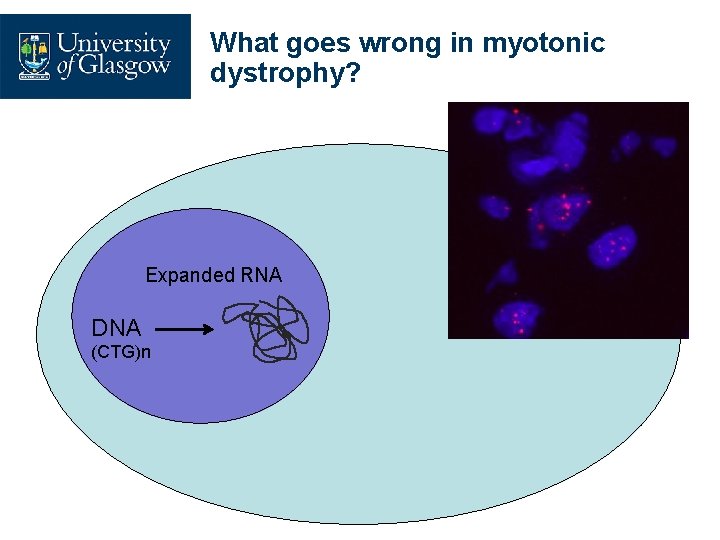
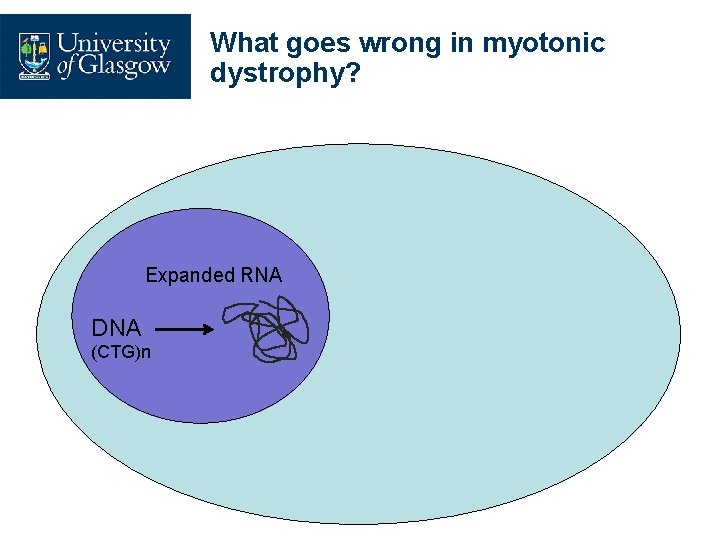
What goes wrong in myotonic dystrophy? Expanded RNA DNA (CTG)n

What goes wrong in myotonic dystrophy? Expanded RNA DNA (CTG)n

What goes wrong in myotonic dystrophy? Expanded RNA DNA (CTG)n

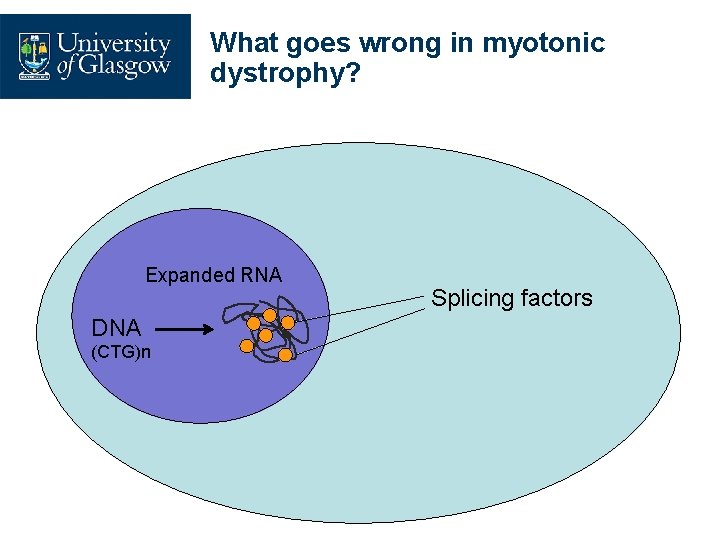
What goes wrong in myotonic dystrophy? Expanded RNA DNA (CTG)n Splicing factors

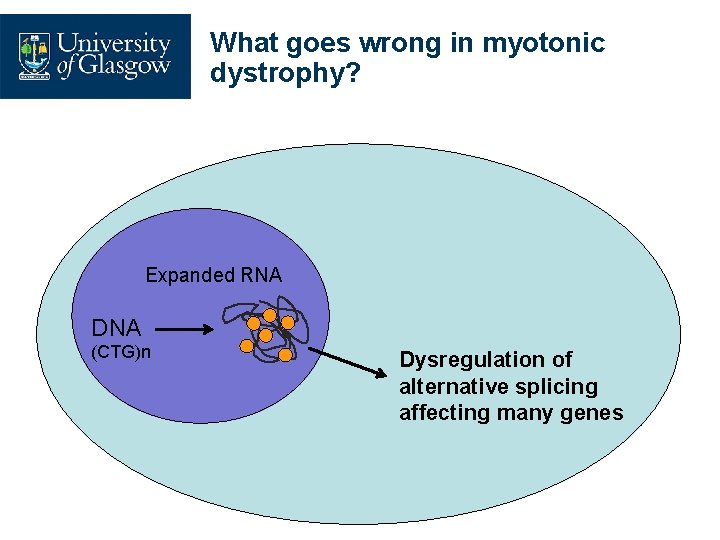
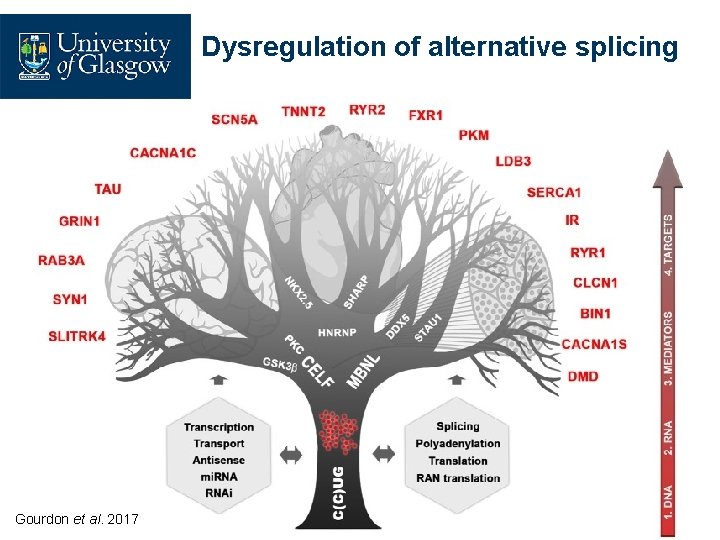
What goes wrong in myotonic dystrophy? Expanded RNA DNA (CTG)n Dysregulation of alternative splicing affecting many genes

Dysregulation of alternative splicing Gourdon et al. 2017

Myotonic dystrophy is a multisystem disorder

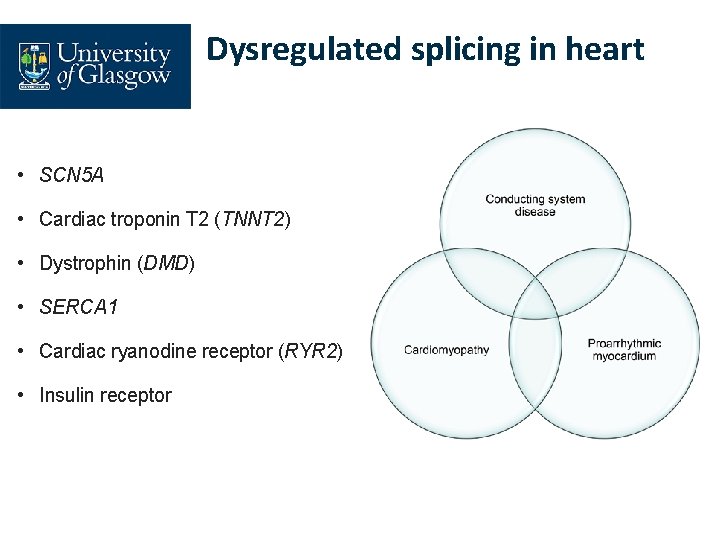
Dysregulated splicing in heart • SCN 5 A • Cardiac troponin T 2 (TNNT 2) • Dystrophin (DMD) • SERCA 1 • Cardiac ryanodine receptor (RYR 2) • Insulin receptor

Dysregulated splicing in heart • SCN 5 A • Cardiac troponin T 2 (TNNT 2) • Dystrophin (DMD) • SERCA 1 • Cardiac ryanodine receptor (RYR 2) • Insulin receptor

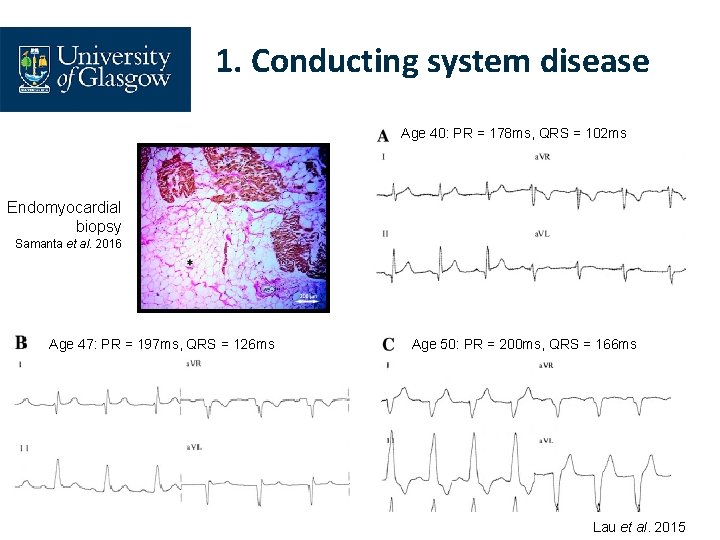
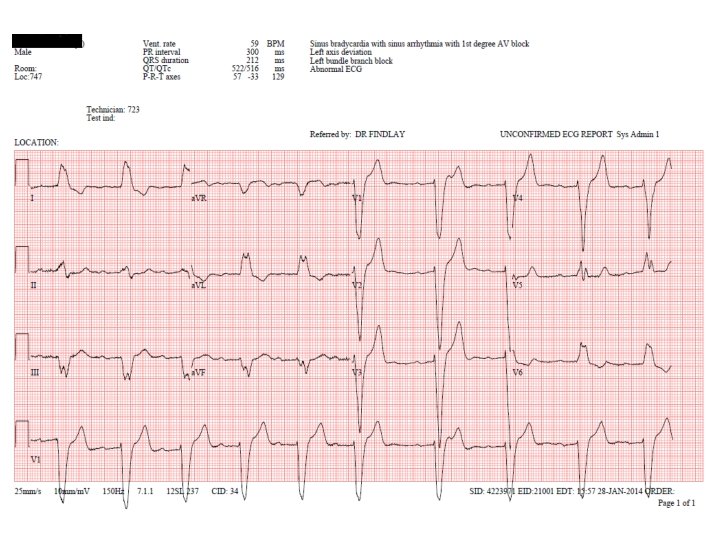
1. Conducting system disease Age 40: PR = 178 ms, QRS = 102 ms Endomyocardial biopsy Samanta et al. 2016 Age 47: PR = 197 ms, QRS = 126 ms Age 50: PR = 200 ms, QRS = 166 ms Lau et al. 2015


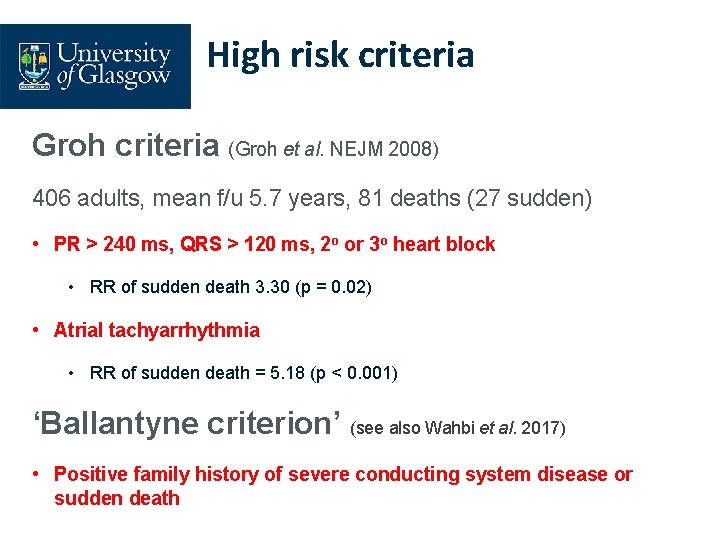
High risk criteria Groh criteria (Groh et al. NEJM 2008) 406 adults, mean f/u 5. 7 years, 81 deaths (27 sudden) • PR > 240 ms, QRS > 120 ms, 2 o or 3 o heart block • RR of sudden death 3. 30 (p = 0. 02) • Atrial tachyarrhythmia • RR of sudden death = 5. 18 (p < 0. 001) ‘Ballantyne criterion’ (see also Wahbi et al. 2017) • Positive family history of severe conducting system disease or sudden death

The case for pacing • 1, 388 patients over median 10 year follow-up (Wahbi et al. 2017) • 8/17 arrhythmic deaths were potentially PPM treatable (5 x asystole, 1 x 3 o AV block, 2 x EMD) • 143 (> 10%) had major conduction defects (99 x 3 o AV block, 32 x 2 o AV block, 12 x sinus node dysfunction) • Electrophysiology in 100 consecutive patients (Laurent et al. 2011) • 49 given PPM for HV ≥ 70 ms • 46 met Groh criteria (32 of whom got pacemakers) • Only one sudden death in ~ 6 years. A “ 6 -fold reduction”

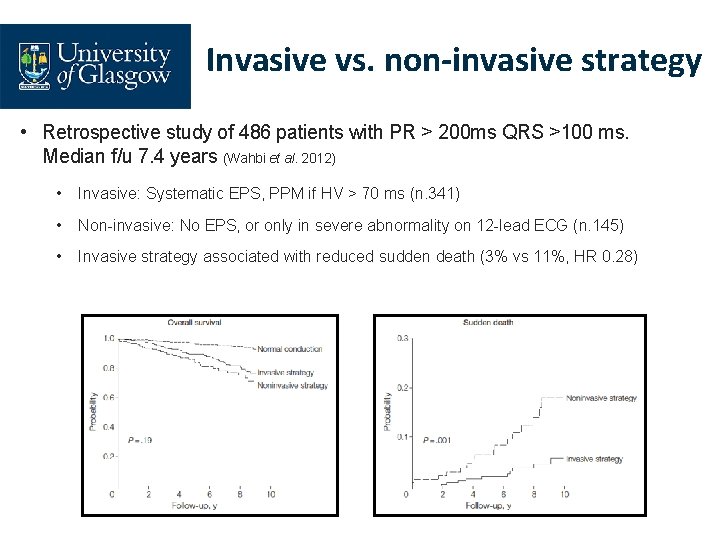
Invasive vs. non-invasive strategy • Retrospective study of 486 patients with PR > 200 ms QRS >100 ms. Median f/u 7. 4 years (Wahbi et al. 2012) • Invasive: Systematic EPS, PPM if HV > 70 ms (n. 341) • Non-invasive: No EPS, or only in severe abnormality on 12 -lead ECG (n. 145) • Invasive strategy associated with reduced sudden death (3% vs 11%, HR 0. 28)

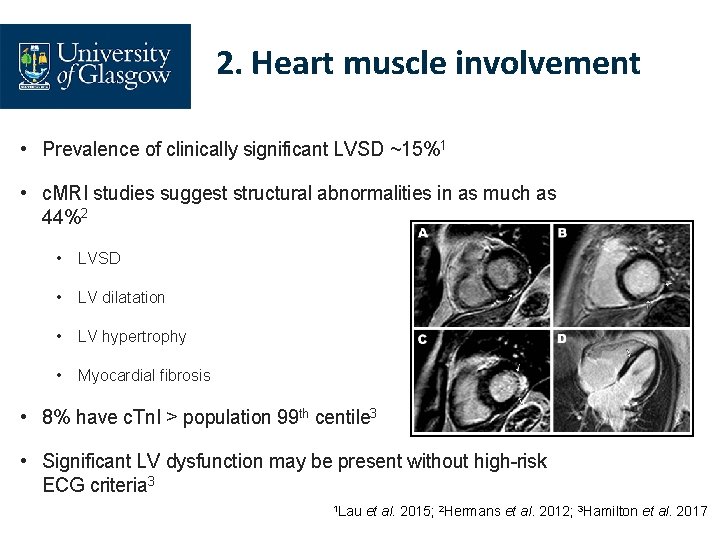
2. Heart muscle involvement • Prevalence of clinically significant LVSD ~15%1 • c. MRI studies suggest structural abnormalities in as much as 44%2 • LVSD • LV dilatation • LV hypertrophy • Myocardial fibrosis • 8% have c. Tn. I > population 99 th centile 3 • Significant LV dysfunction may be present without high-risk ECG criteria 3 1 Lau et al. 2015; 2 Hermans et al. 2012; 3 Hamilton et al. 2017

Management of LVSD in myotonic dystrophy • No RCT data (to my knowledge) • Myotonic Dystrophy Foundation (MDF) consensus care recommendations (2018) suggest simply follow generic ACCF/AHA guidelines for management of heart failure • But (presumably) extreme caution needed with beta-blockers?

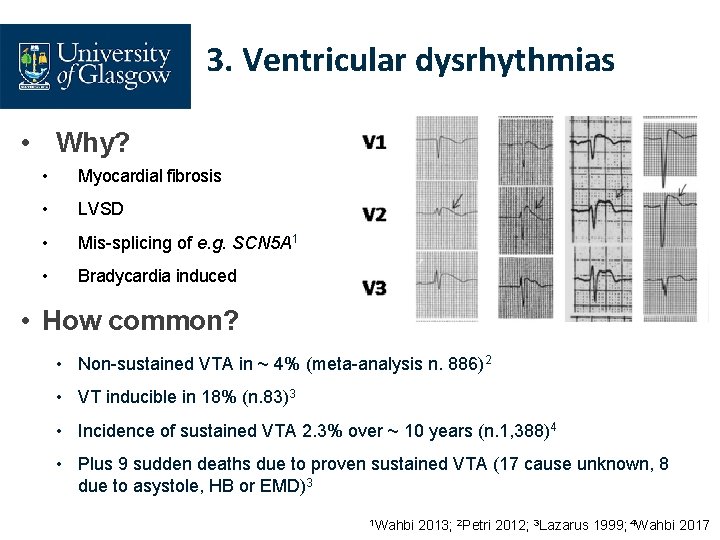
3. Ventricular dysrhythmias • Why? • Myocardial fibrosis • LVSD • Mis-splicing of e. g. SCN 5 A 1 • Bradycardia induced • How common? • Non-sustained VTA in ~ 4% (meta-analysis n. 886)2 • VT inducible in 18% (n. 83)3 • Incidence of sustained VTA 2. 3% over ~ 10 years (n. 1, 388)4 • Plus 9 sudden deaths due to proven sustained VTA (17 cause unknown, 8 due to asystole, HB or EMD)3 1 Wahbi 2013; 2 Petri 2012; 3 Lazarus 1999; 4 Wahbi 2017

Risk factors for VTA and sudden death (Wahbi et al. 2017) Outcome Risk factors Overall survival Age; male sex; atrial fibrillation (AF); syncope; heart rate; PR and QRS intervals; 1 st degree AV block; left BBB, right BBB, and LVEF <50% Sudden death Age; family history of SD; non-sustained VT; AF; PR and QRS intervals; 1 st degree AV block; left BBB Sustained VTA Coronary artery disease; AF; non-sustained VTA; QRS interval; left and right BBB; LVEF <50% Major conduction defects Age; male sex; AF; LVEF <50%; any conduction defect on the ECG • No disease-specific guideline for ICD implantation • Authors suggest history of VTA, severe LVSD, type 1 Brugada ECG should prompt consideration

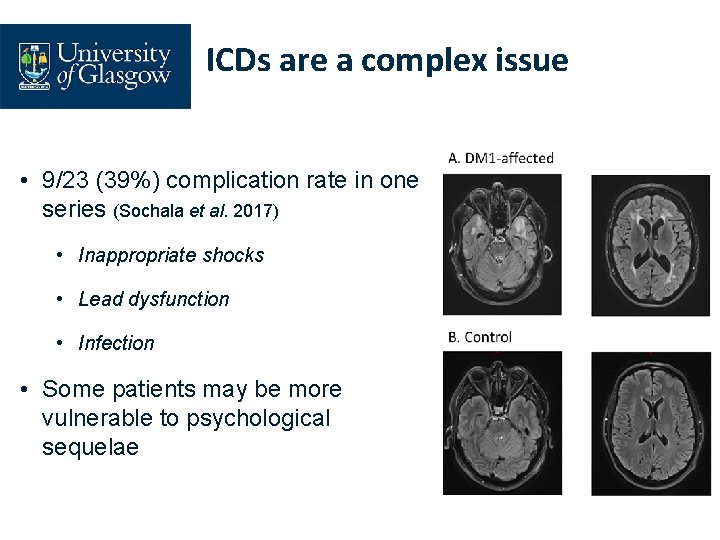
ICDs are a complex issue • 9/23 (39%) complication rate in one series (Sochala et al. 2017) • Inappropriate shocks • Lead dysfunction • Infection • Some patients may be more vulnerable to psychological sequelae

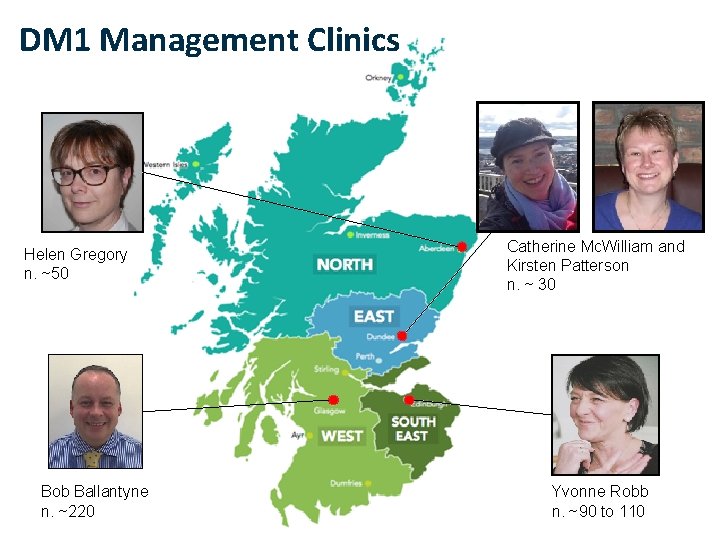
DM 1 Management Clinics Helen Gregory n. ~50 Bob Ballantyne n. ~220 Catherine Mc. William and Kirsten Patterson n. ~ 30 Yvonne Robb n. ~90 to 110

Key documents Brand new Myotonic Dystrophy Foundation (MDF) 2018 Feingold et al. 2017

Consensus approach to management • All patients with a diagnosis of myotonic dystrophy should have annual ECG and symptomatic review at least • All patients with ECG abnormality, or palpitations or syncope should be referred to cardiologist • 24 Holter • MDF recommends 3 to 5 yearly if abnormal ECG (more frequent if clinically indicated) • AHA guideline suggests 2 to 4 yearly for all • Echo • MDF recommends at diagnosis then 3 to 5 yearly (more frequent if clinically indicated) • AHA guideline suggests 2 to 4 yearly for all

Consensus approach to management • Treatment of LVSD • According to American Heart Association Guidelines for Management of Heart Failure? • ICD placement • ACC/AHA Guideline for Device-based therapy • Patients with devices should remain under formal cardiology review (i. e. pacemaker checks alone are not adequate) • Is there a role for invasive electrophysiology?

Summary • Cardiac phenotype in myotonic dystrophy is complex • Proactive screening and early intervention for conducting system disease is life-saving • Optimal strategy for ICD implantation not fully elucidated • Severe LVSD, non-sustained VT, Brugada-like ECG • Scottish DM 1 cohort represent an excellent opportunity for audit, research and service-improvement work

The Scottish Myotonic Dystrophy Consortium Cheryl Longman Douglas Wilcox Alison Wilcox Richard Petty Yvonne Robb Mark Hamilton Anne-Marie Taylor Maria Elena Farrugia Helen Gregory Alexis Duncan Catherine Mc. William John Dean Bob Ballantyne Lorna Mac. Leish Programme Manager: Hugh Kennedy Programme Support Officer: Laura Craig Monika Rahman Anne Mc. Keown Kirsten Patterson Sarah Cumming Darren G. Monckton