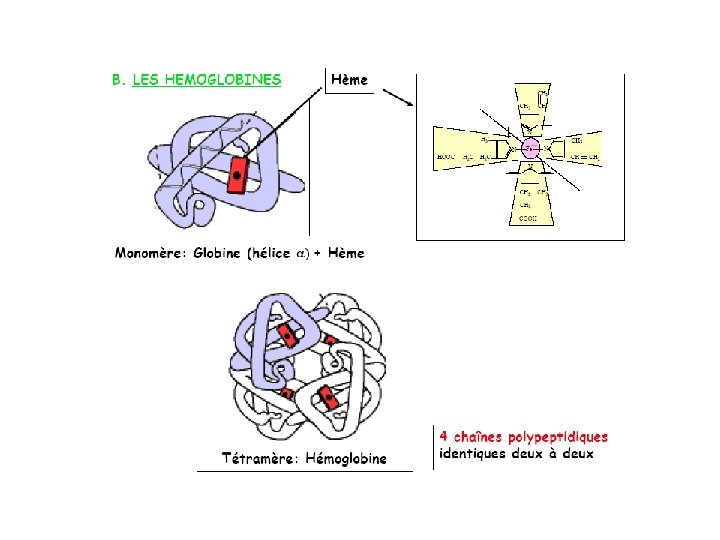
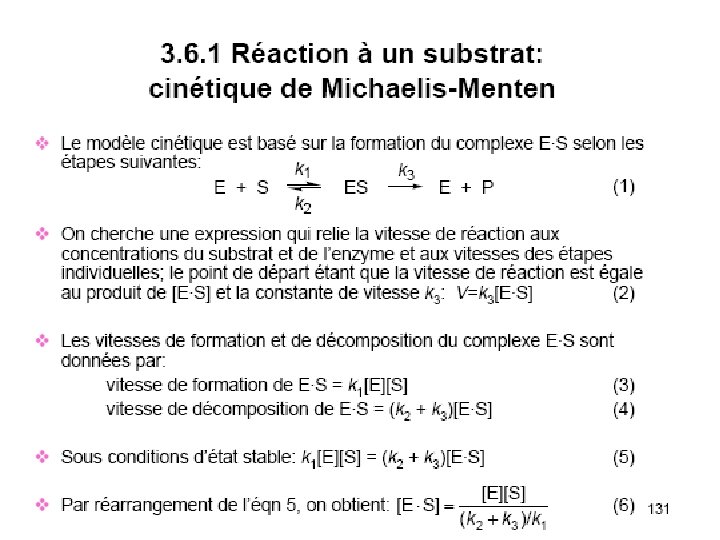
Myoglobine hmoglobine 153 aas 2 2 141 aas
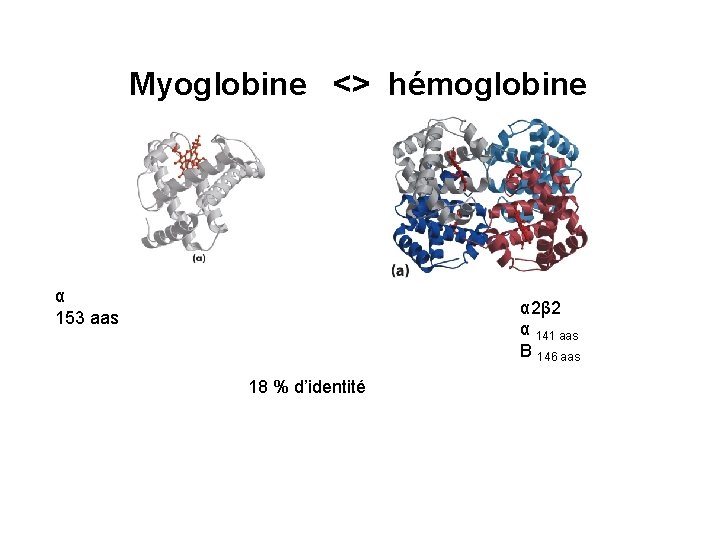
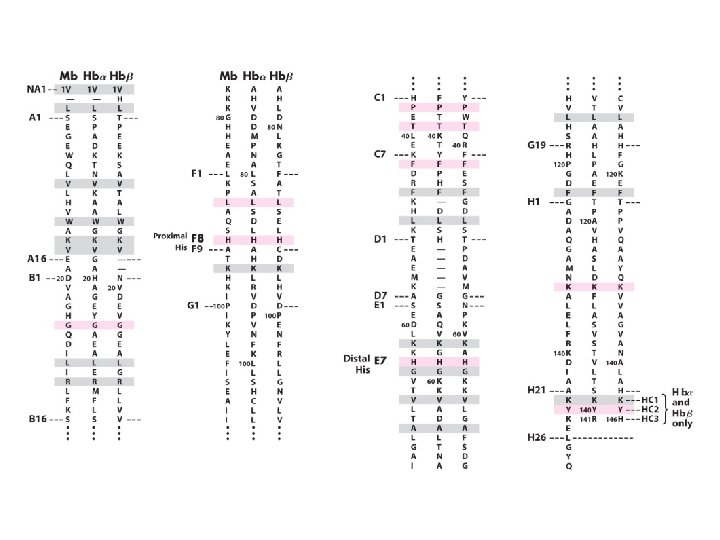
Myoglobine <> hémoglobine α 153 aas α 2β 2 α 141 aas Β 146 aas 18 % d’identité
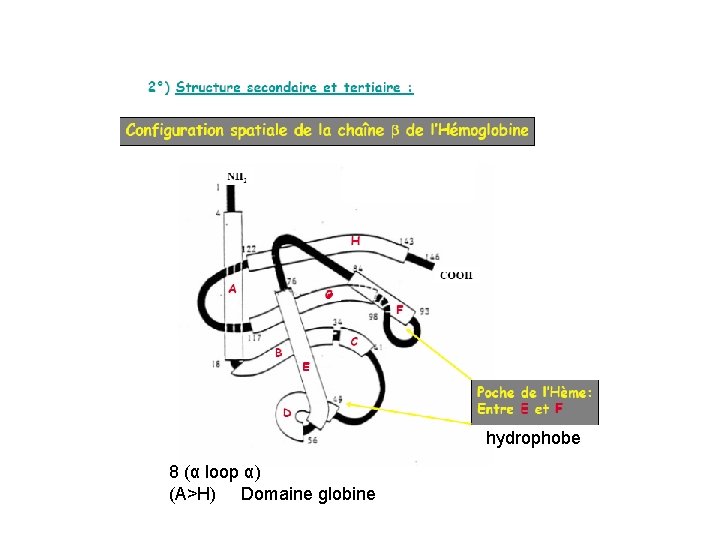
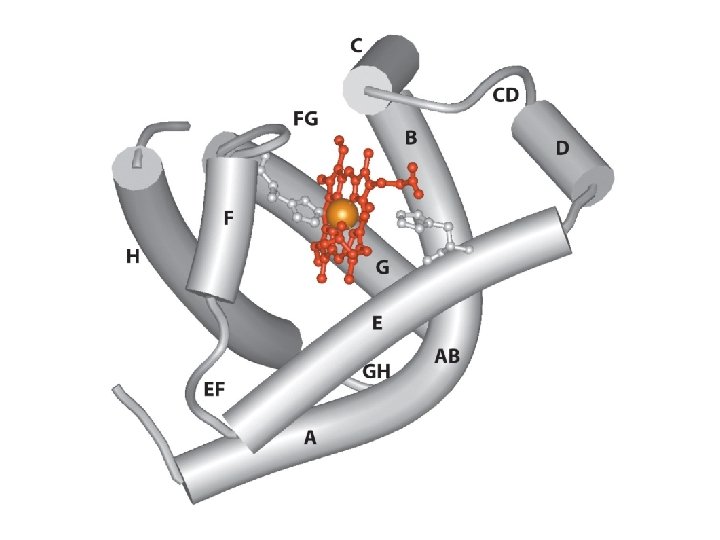
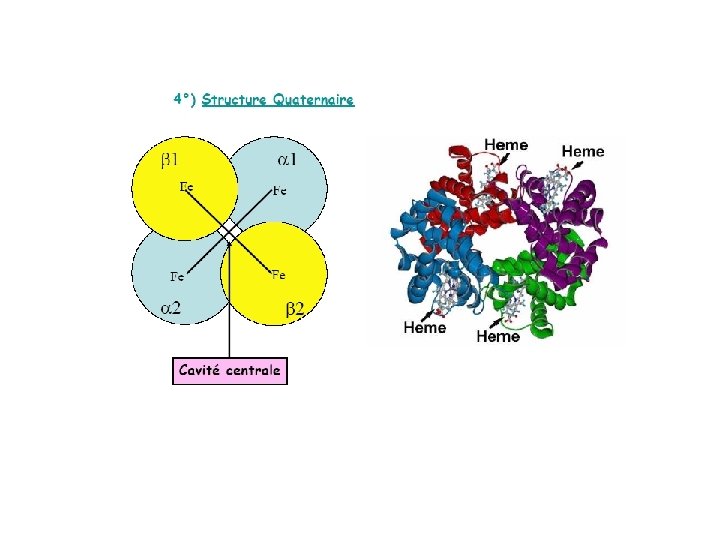
hydrophobe 8 (α loop α) (A>H) Domaine globine
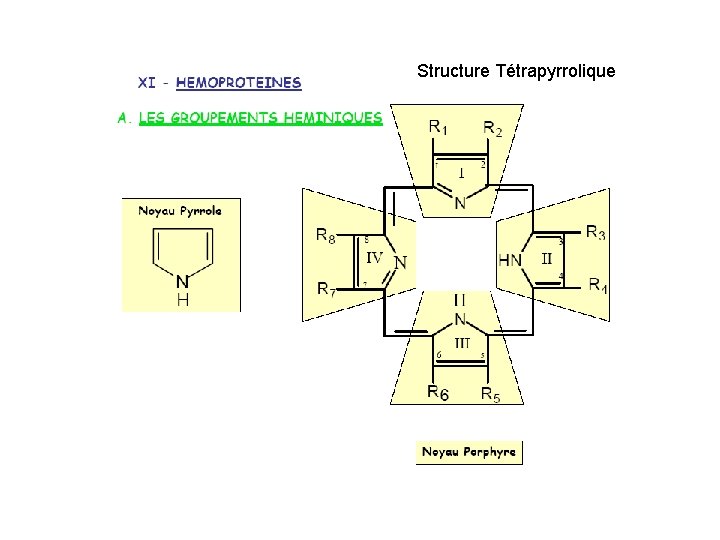
Structure Tétrapyrrolique
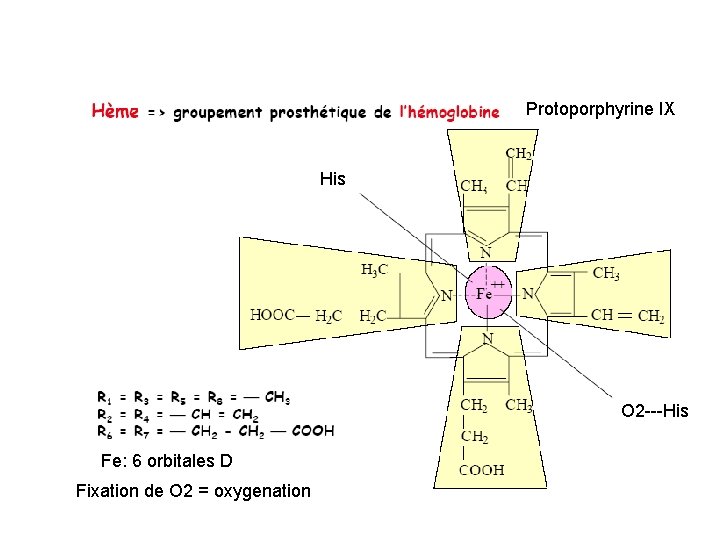
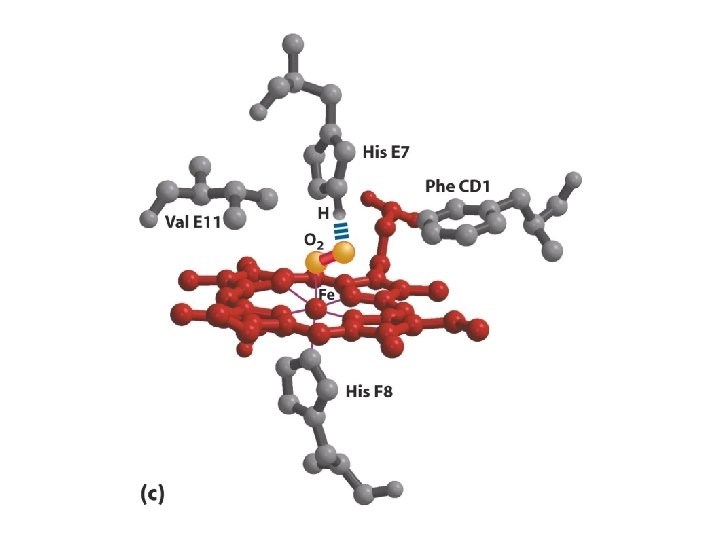
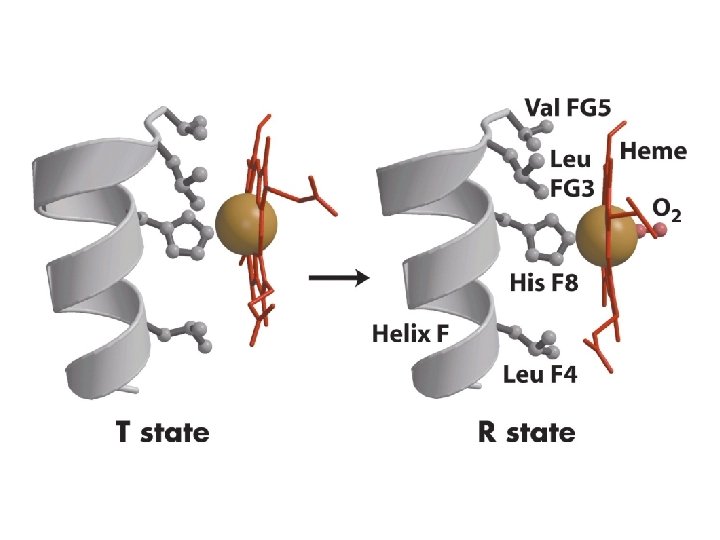
Protoporphyrine IX His O 2 ---His Fe: 6 orbitales D Fixation de O 2 = oxygenation
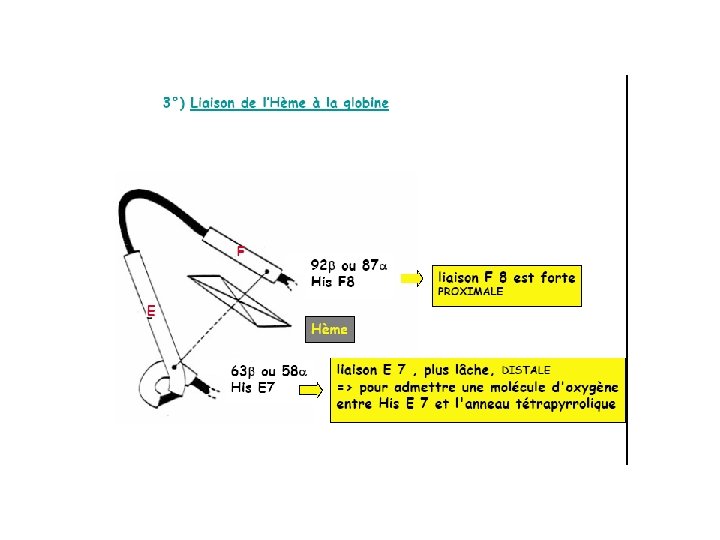
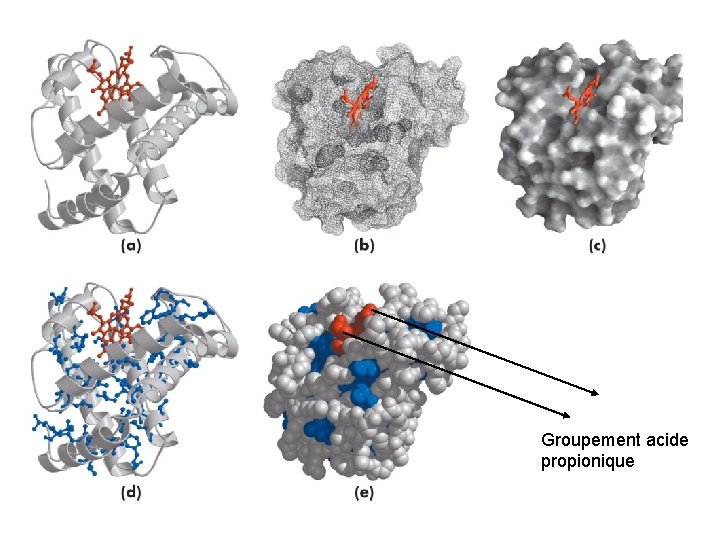
Groupement acide propionique
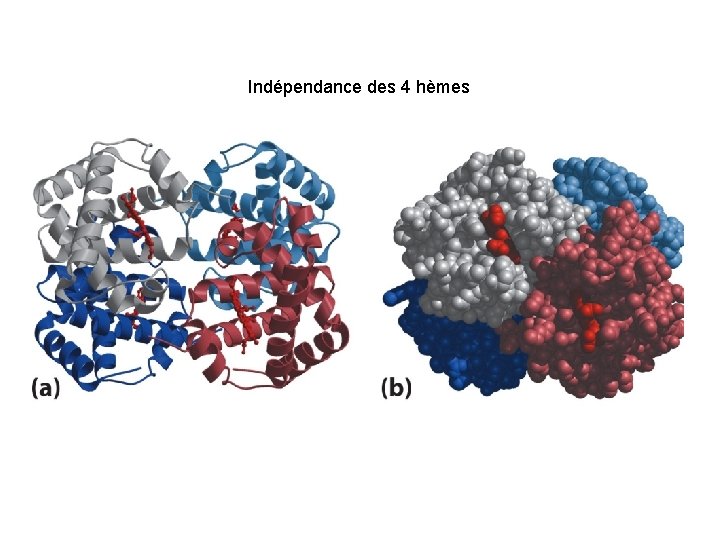
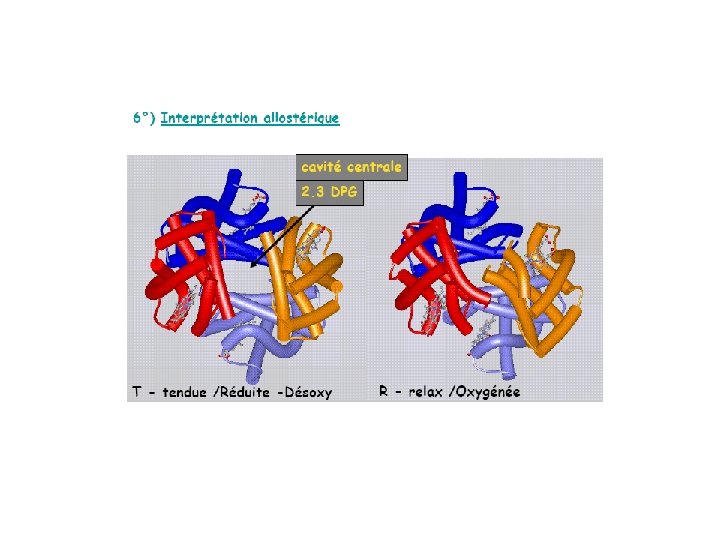
Indépendance des 4 hèmes

O 2 Gaz hydrophobe>> peu soluble Mb et Hb servent de transporteurs
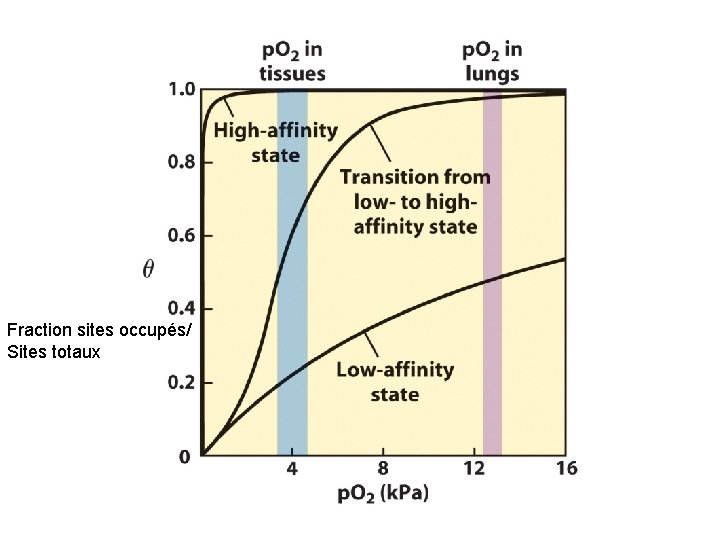
Fraction sites occupés/ Sites totaux

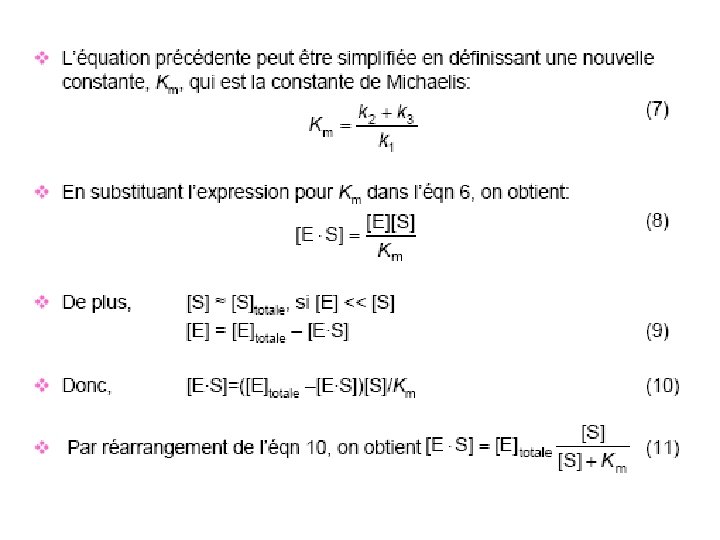
Pourquoi structure quaternaire ? ? Hb acquiert des propriétés plus complexe que la myoglobine - son affinité est adapté à l’état de l’environement (interaction de molécules) Proton (effet Bohr) 2, 3 BPG
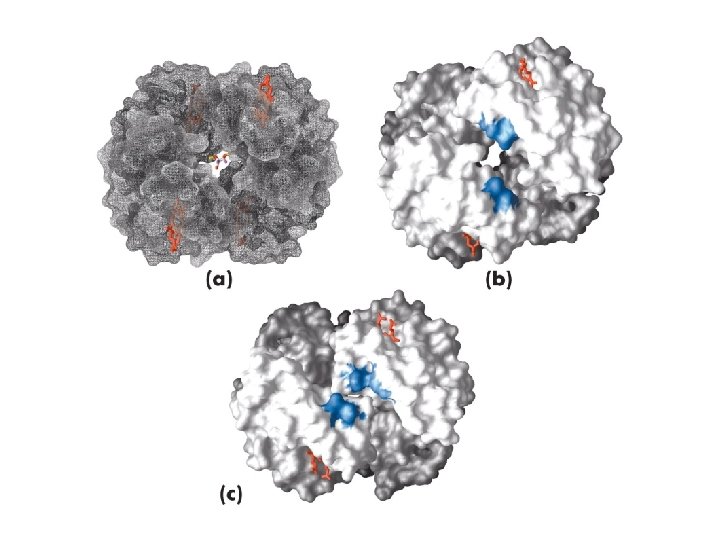
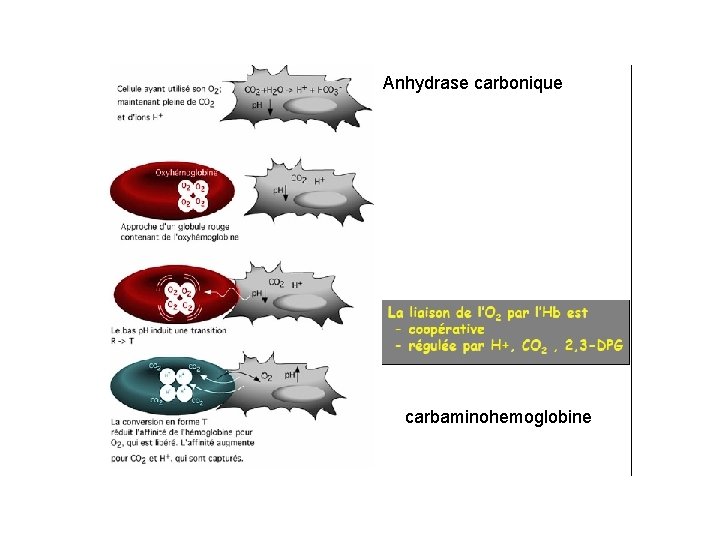
Anhydrase carbonique carbaminohemoglobine
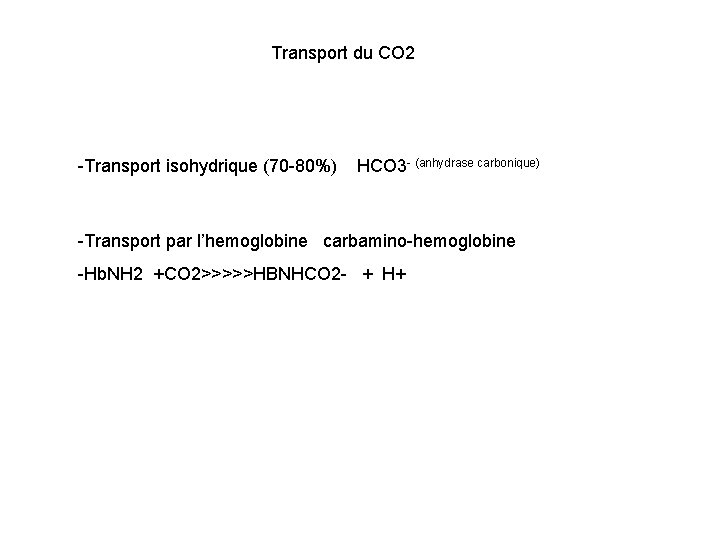
Transport du CO 2 -Transport isohydrique (70 -80%) HCO 3 - (anhydrase carbonique) -Transport par l’hemoglobine carbamino-hemoglobine -Hb. NH 2 +CO 2>>>>>HBNHCO 2 - + H+
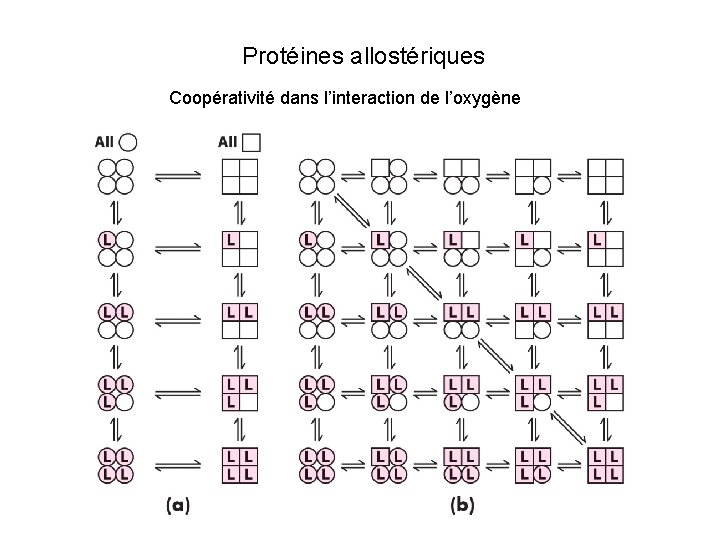
Protéines allostériques Coopérativité dans l’interaction de l’oxygène






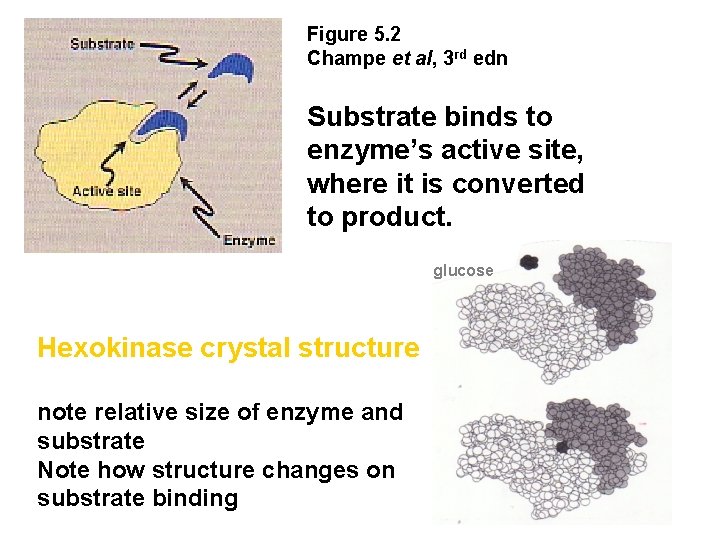
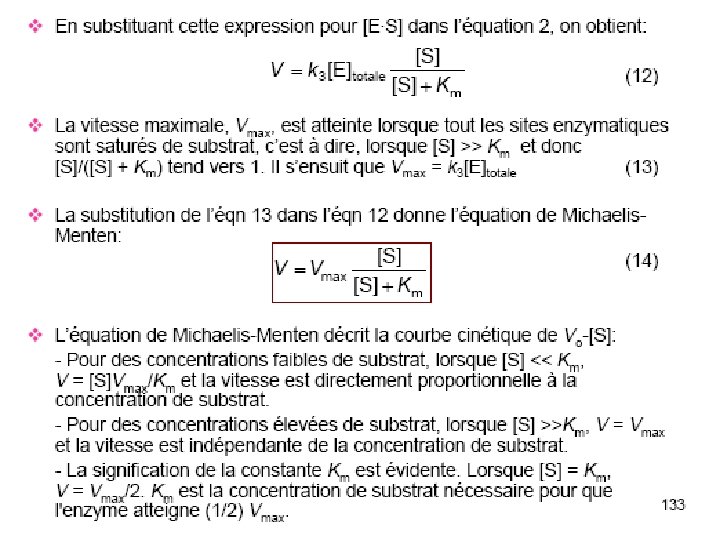
Figure 5. 2 Champe et al, 3 rd edn Substrate binds to enzyme’s active site, where it is converted to product. glucose Hexokinase crystal structure note relative size of enzyme and substrate Note how structure changes on substrate binding
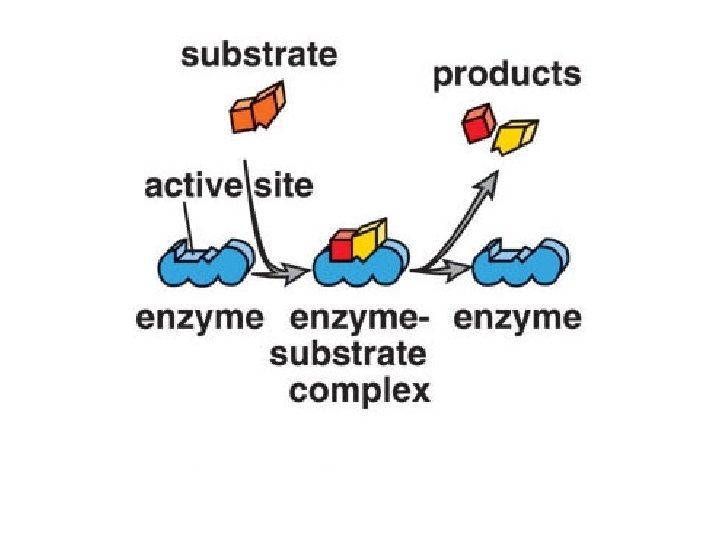
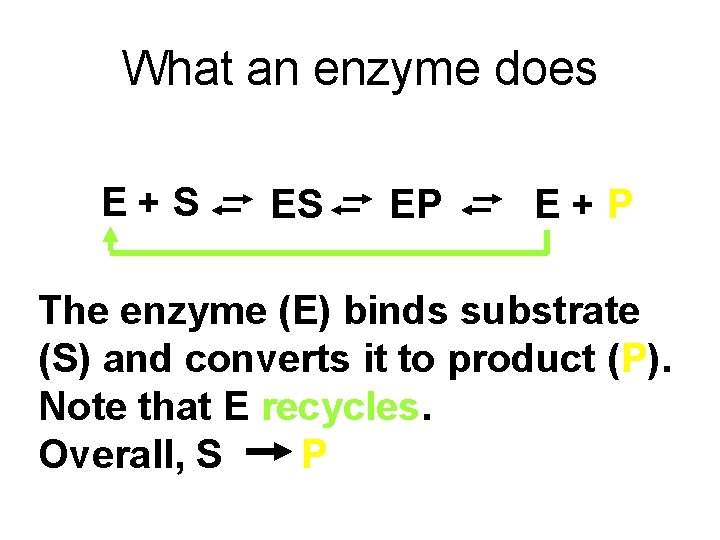
What an enzyme does E+S ES EP E+P The enzyme (E) binds substrate (S) and converts it to product (P). Note that E recycles. Overall, S P
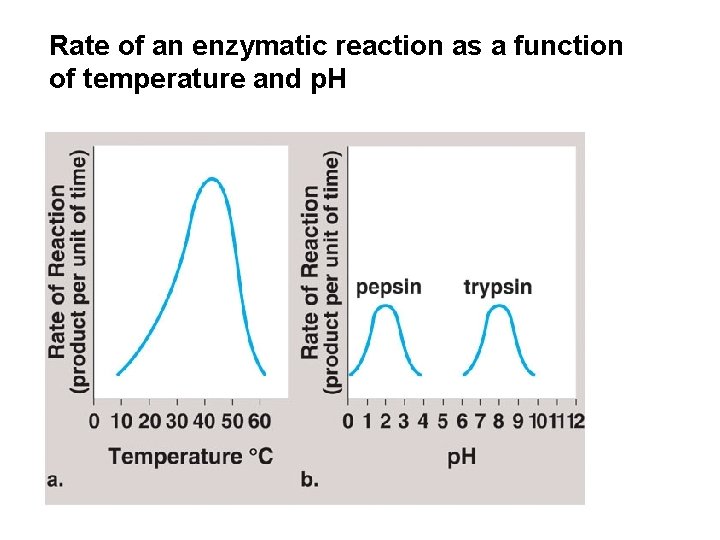
Rate of an enzymatic reaction as a function of temperature and p. H
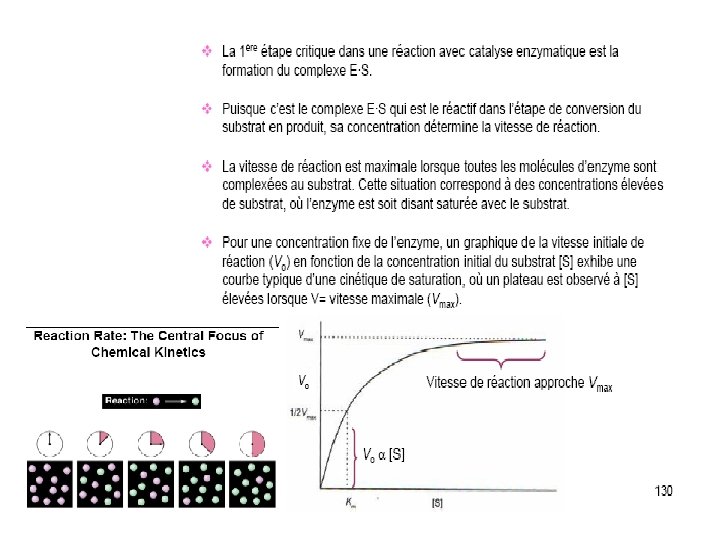
![Km – a measure of E-S affinity • Km = [E]. [S] [ES] • Km – a measure of E-S affinity • Km = [E]. [S] [ES] •](http://slidetodoc.com/presentation_image_h2/f496bdec69da8de281b3b3a181148d23/image-40.jpg)
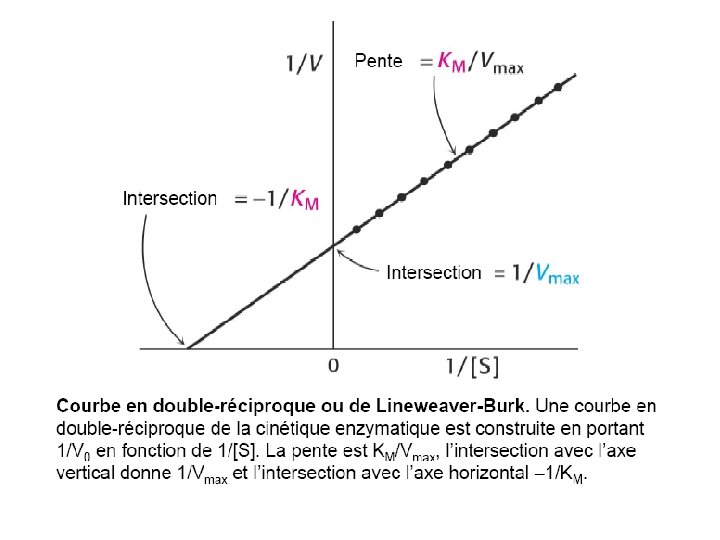
Km – a measure of E-S affinity • Km = [E]. [S] [ES] • When E is ½ saturated with S (vo= ½ Vmax) • Then [ES] = [E] • and Km = [S] • Units are M (mol/l) Km is the substrate concentration at which vo = ½ Vmax. low Km = high affinity high Km = low affinity
- Slides: 41