Myers Synthesis of Dynemicin A Andrew G Myers



- Slides: 14

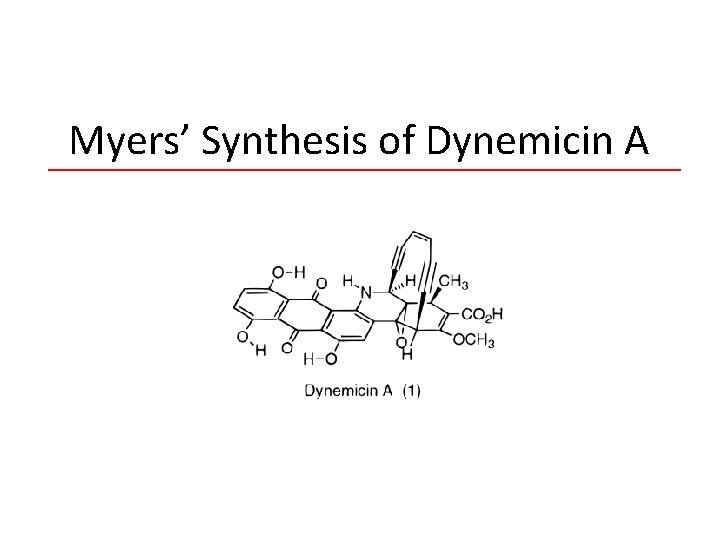
Myers’ Synthesis of Dynemicin A

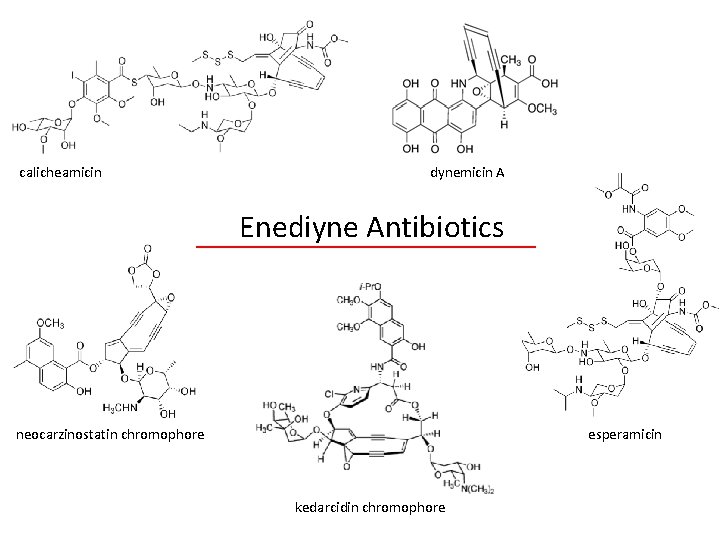
Andrew G. Myers • • 140 publications to date (1981) B. S. at MIT – undergraduate research with W. R. Roush. (1981 -86) Graduate studies and brief post-doctoral work under E. J. Corey. (1986) Began independent career – California Institute of Technology – By 1994, became full professor. • (1998) Moved to Harvard – 2007 -2010, Served as chair of the chemistry department. • Dr. Meyer’s research focuses on the synthesis of medically/biologically active compounds for human medicine development. – Convergent, practical, and scalable syntheses. – Enediyne antibiotics synthesized: Neocarzinostatin chromophore, N 1999 A 2, kedarcidin chromophore, dynemicin A. – Methodology development.

calicheamicin dynemicin A Enediyne Antibiotics neocarzinostatin chromophore esperamicin kedarcidin chromophore

Bioactivity – Intercalation and cleavage of DNA -Enediyne antibiotics cleave both strands of DNA! -Irreversible damage -Anti-tumor activity

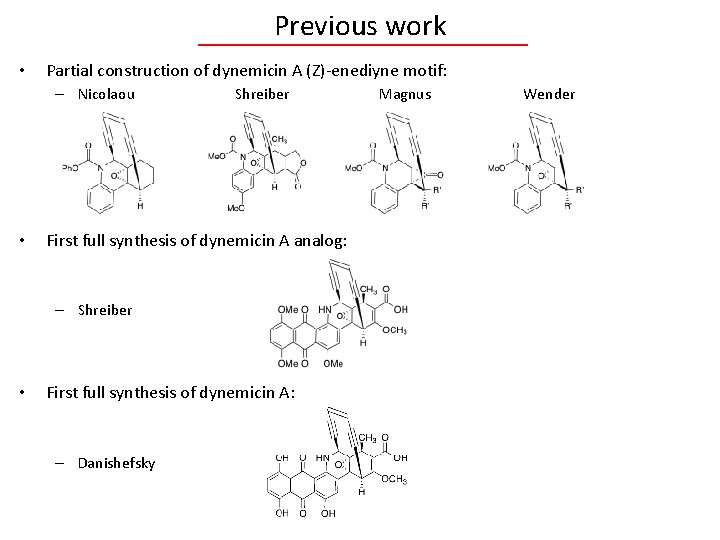
Previous work • Partial construction of dynemicin A (Z)-enediyne motif: – Nicolaou • Shreiber First full synthesis of dynemicin A analog: – Shreiber • First full synthesis of dynemicin A: – Danishefsky Magnus Wender

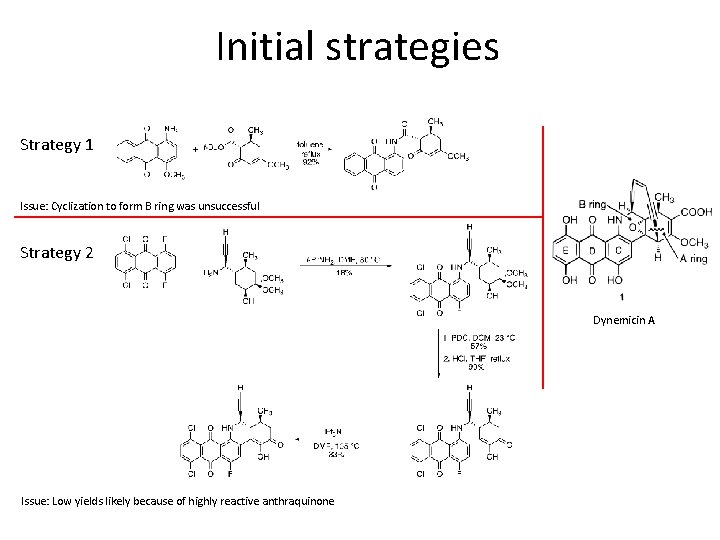
Initial strategies Strategy 1 Issue: Cyclization to form B ring was unsuccessful Strategy 2 Dynemicin A Issue: Low yields likely because of highly reactive anthraquinone

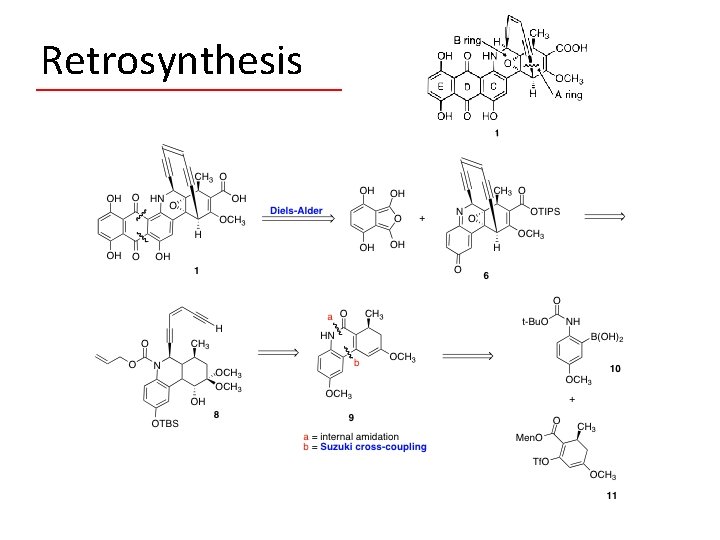
Retrosynthesis

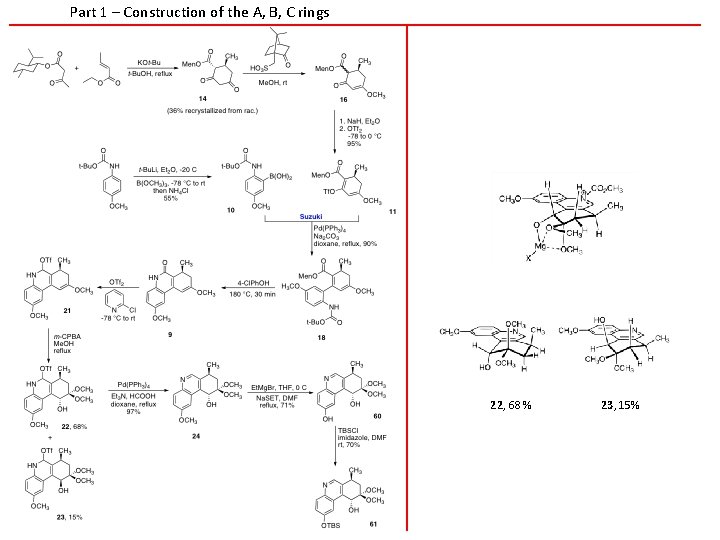
Part 1 – Construction of the A, B, C rings 22, 68 % 23, 15%

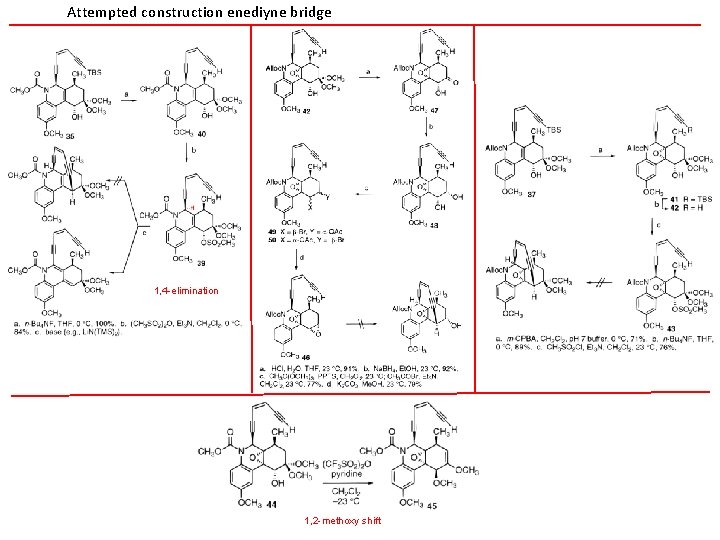
Attempted construction enediyne bridge 1, 4 -elimination 1, 2 -methoxy shift

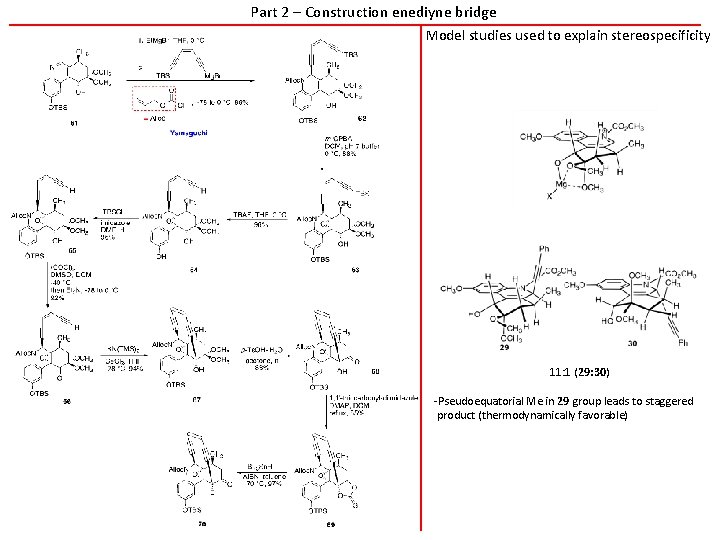
Part 2 – Construction enediyne bridge Model studies used to explain stereospecificity 11: 1 (29: 30) -Pseudoequatorial Me in 29 group leads to staggered product (thermodynamically favorable)

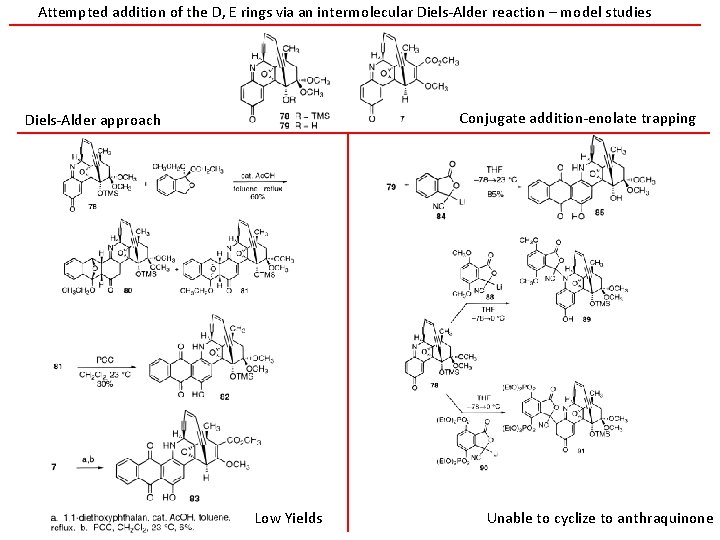
Attempted addition of the D, E rings via an intermolecular Diels-Alder reaction – model studies Conjugate addition-enolate trapping Diels-Alder approach Low Yields Unable to cyclize to anthraquinone

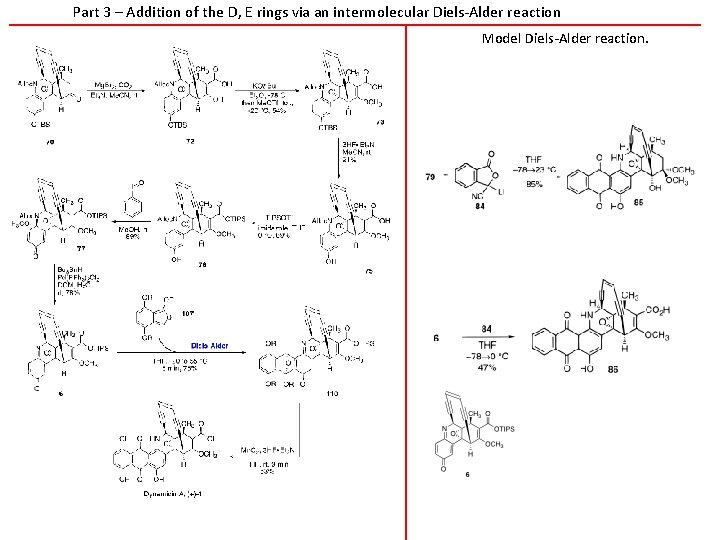
Part 3 – Addition of the D, E rings via an intermolecular Diels-Alder reaction Model Diels-Alder reaction.

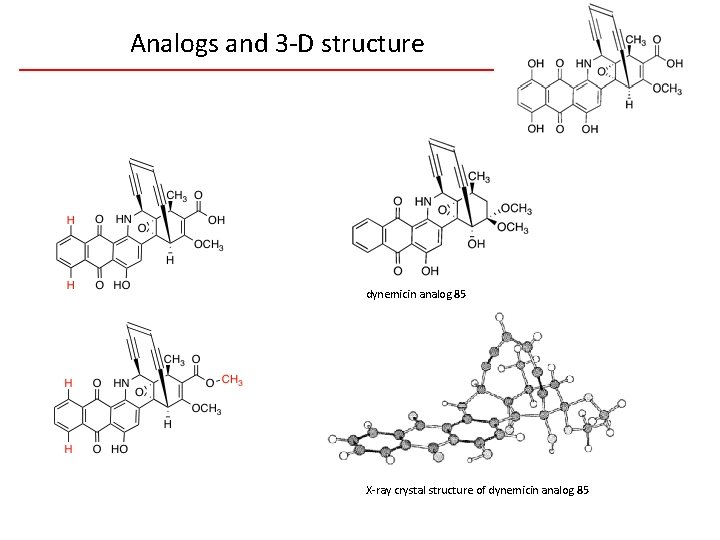
Analogs and 3 -D structure dynemicin analog 85 X-ray crystal structure of dynemicin analog 85

The Myers Group
 Andrew myers cornell
Andrew myers cornell Andrew myers harvard
Andrew myers harvard Tipo de personalidad arquitecto
Tipo de personalidad arquitecto Mr irwin lasher
Mr irwin lasher N vs s myers briggs
N vs s myers briggs Tony robbins myers briggs
Tony robbins myers briggs Wide area network wan fort myers
Wide area network wan fort myers Reader response theory
Reader response theory Mmpi vs myers briggs
Mmpi vs myers briggs Myers' psychology for ap solutions
Myers' psychology for ap solutions Brealey and myers
Brealey and myers Determinants of corporate borrowing
Determinants of corporate borrowing Pig psychology test
Pig psychology test Manber myers
Manber myers Myers briggs dichotomies
Myers briggs dichotomies