MYELIN SHEATH AND ITS FORMATION Adapted from internet




















- Slides: 51

MYELIN SHEATH AND ITS FORMATION • Adapted from internet sources

NERVOUS SYSTEM • The nervous system is comprised of two primary cell types: neurons and glial cells. • These cells communicate with each other to perform important tasks in the nervous system. • The glial cells support neurons structurally and maintain their long-term neuronal integrity, and neurons regulate glial cell behavior. • In this support of neurons, glial cells have become highly specialized. • Glial cells, which can be divided into several types, have various important functions, such as providing structural support, growth support, and insulation around the axon.



• All information both to and from the body must be coordinated and transmitted simultaneously and very quickly. • The brain itself requires extremely fast speeds to operate at even at the simplest level. • How do the biological tissues of our body support such rapid coordination of the brain, limbs, and sensory input? • They do so with nervous system tissue that imitates electrical wiring.

Why must glial cells support neurons? • Neurons are specialized cells that receive and send signals to other cells through fragile and thin cellular extensions called axons. • These axons extend over distances long and short to reach their target, ultimately connecting neurons with other nerve tissue, muscle tissue, or sensory organs. • For example, some motor neurons in the spinal cord have axons that exceed 1 m in length, connecting the spine to the lower limb muscles. • These axons transmit signals to the target muscle in the form of electric impulses called action potentials. • However, the axons alone are not enough to produce rapid conduction of the electric current necessary for this signal to be sent. • Glial cells are the key element for supporting the messages neurons send and receive all over the body

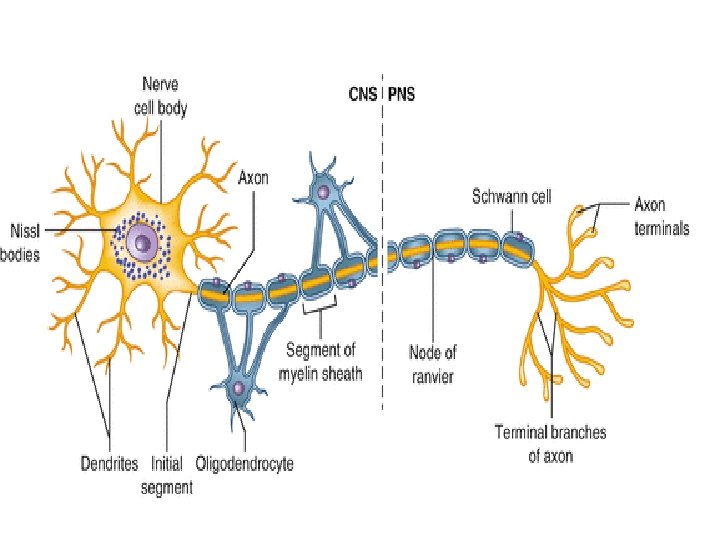
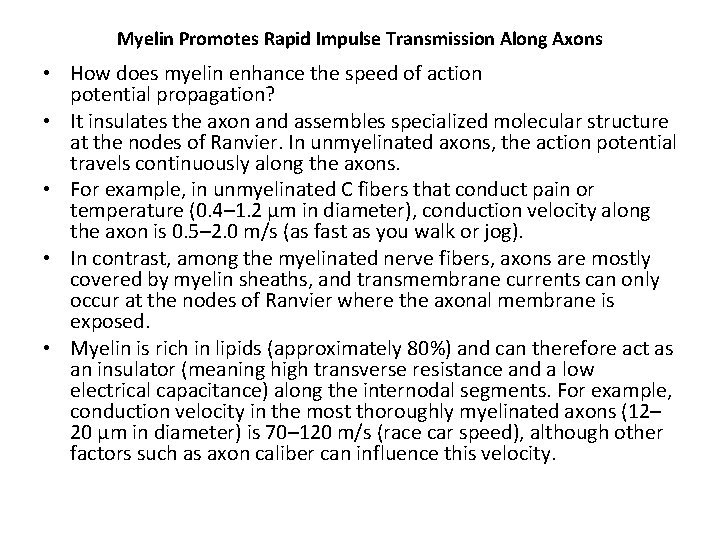
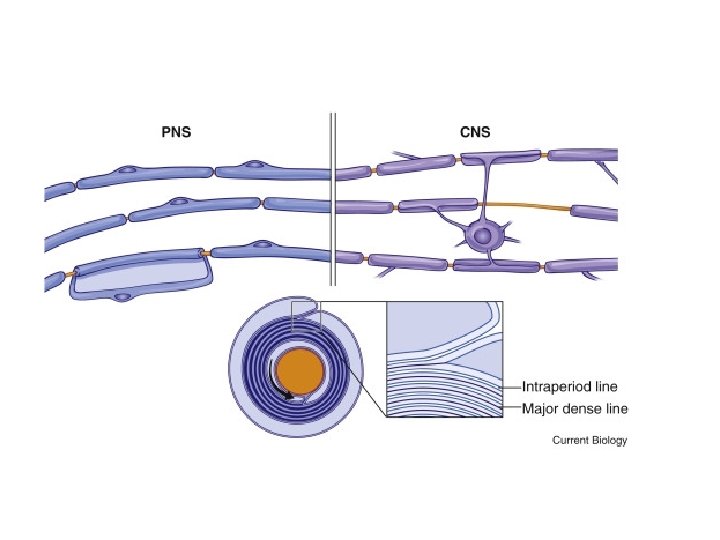
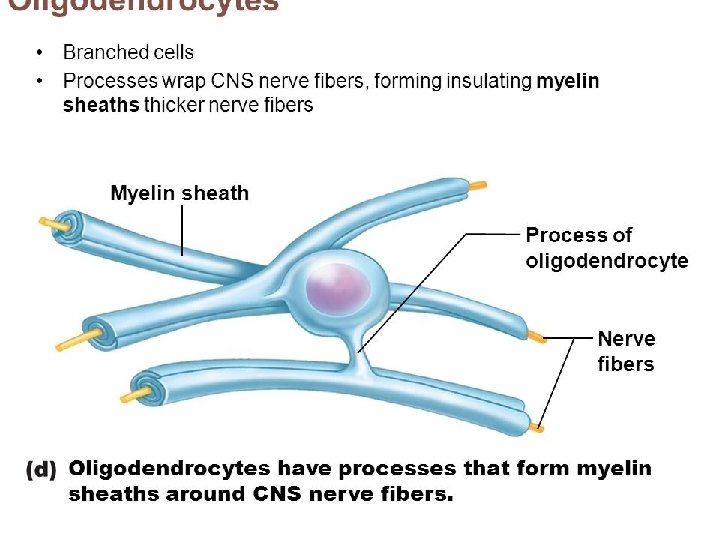
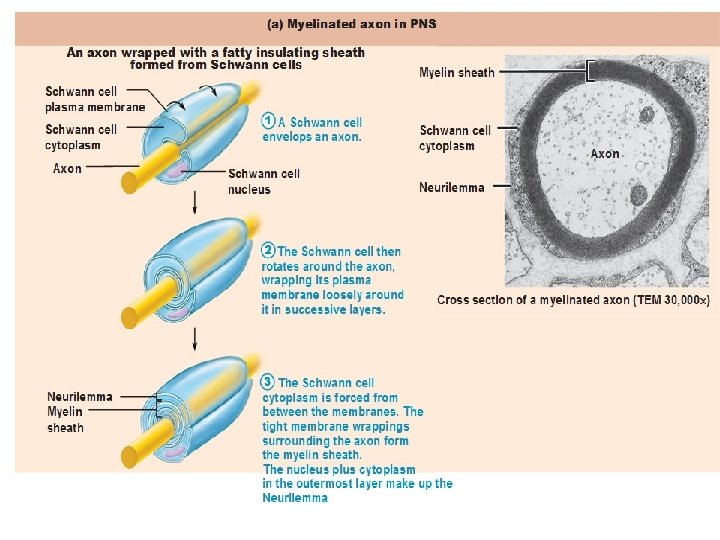
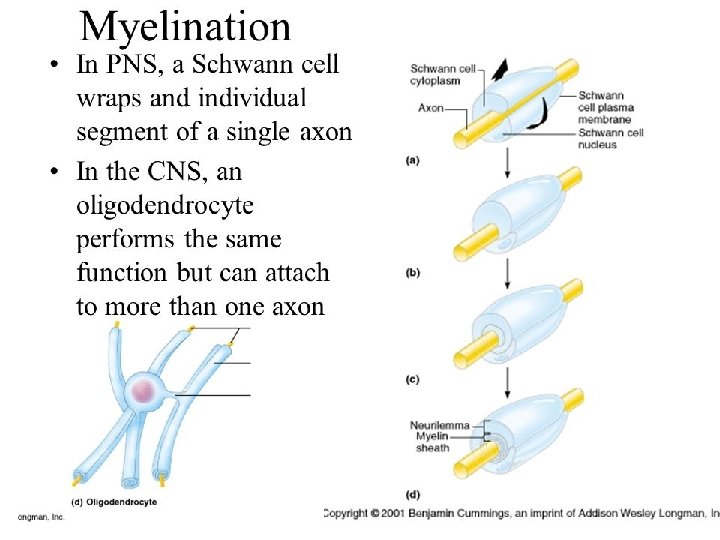
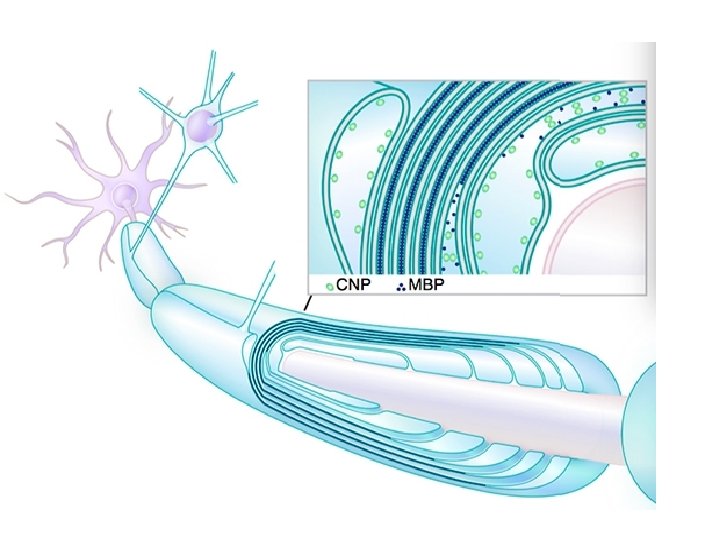
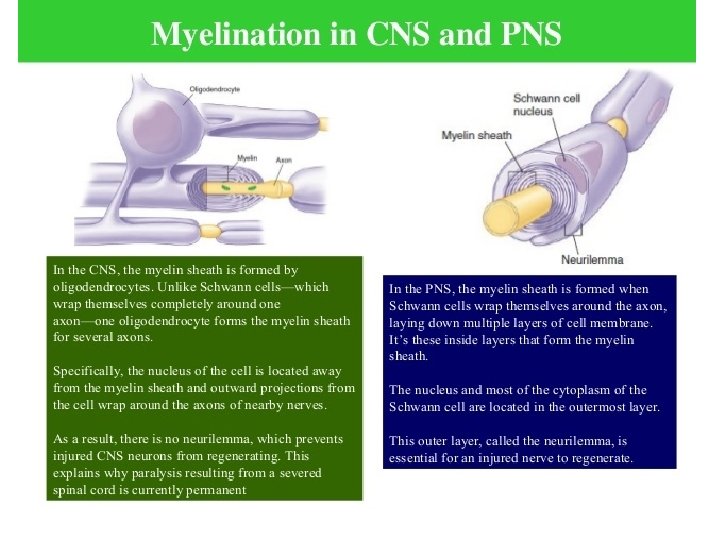
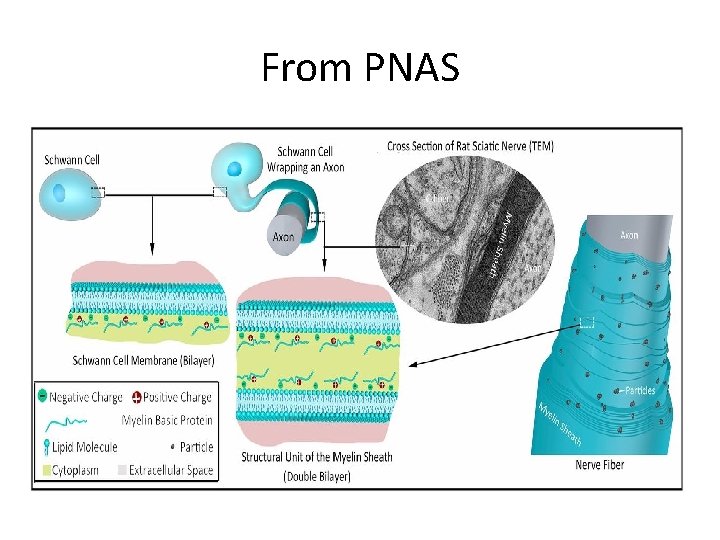
MYELIN SHEATH • Many axons of the Perpheral Nervous System are surrounded by a lipid-rich tubular cover called myelin sheath • External to myelin sheath, a cellular sheath called sheath of schwann or Neurilemma is present. • Only the initial segment of Axon, the axon hillock, and the terminal ramifications are devoid of myelin cover. • Myelin consist of plasma membrane of schwann cells. • The Schwann cell is wrapped around the axon in a spiral fashion for a variable number of times and the thickness of myelin depends on how many wrappings are made. . • Inner leaflets of plasma membrane fuse to form major dense lines observed in myelin; fusion of outer leaflets results in intraperiod lines of myelin. • It is the fused plasma membrane, ie the major dense lines and the intraperiod lines and the material between these lineswhich constitute the myelin.


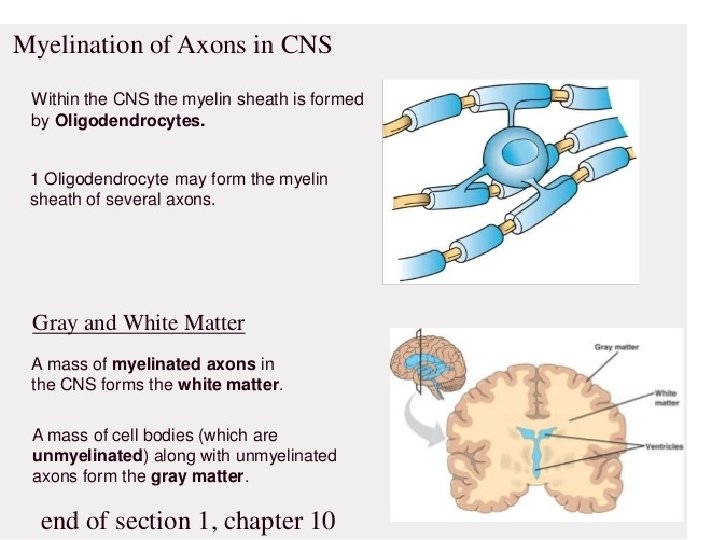
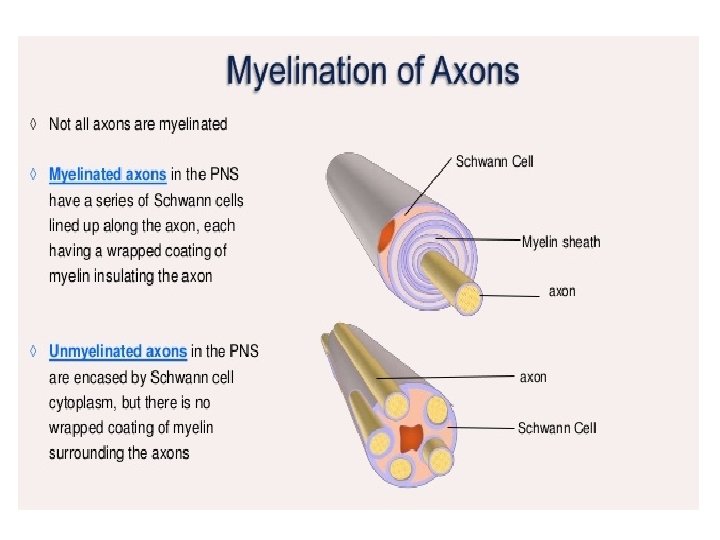
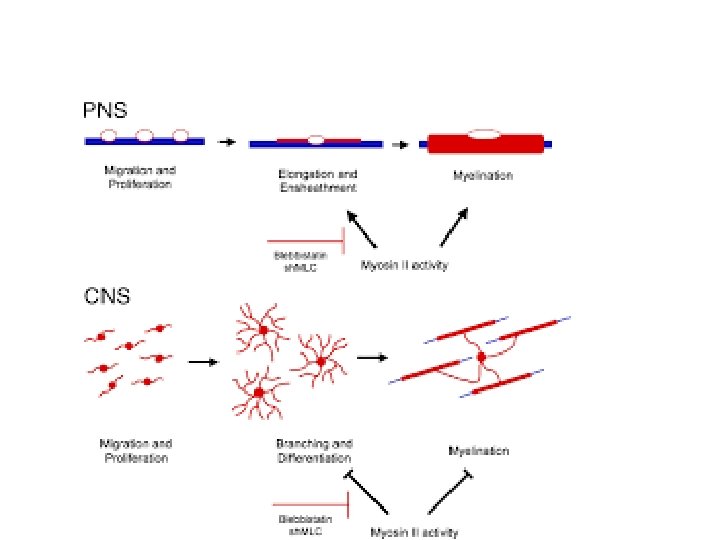
What exactly is myelin? • Myelin is a concentrically laminated membrane structure surrounding an axon around which lamellae (or cellular protrusions) repeat radially at a period of about 12 nm (Waxman, Kocsis & Stys 1995; Sherman & Brophy 2005). • The myelin lamella is formed by fusion of the apposed inner leaflets of the plasma membrane in glial cells, with no intervening cytoplasm. • Depending on the location, different glial cell types make myelin in a different manner. • Schwann cells make myelin in the peripheral nervous system (PNS: nerves) and oligodendrocytes in the central nervous system (CNS: brain and spinal cord). • In the PNS, one Schwann cell forms a single myelin sheath . • By contrast, in the CNS, the oligodendrocyte sends cell processes to myelinate multiple segments on many axons. • Although there are several molecular or morphological differences between nerve fibers in the PNS and CNS, the basic myelin sheath arrangement and the electrophysiological characteristics are essentially the same.


Myelin Promotes Rapid Impulse Transmission Along Axons • How does myelin enhance the speed of action potential propagation? • It insulates the axon and assembles specialized molecular structure at the nodes of Ranvier. In unmyelinated axons, the action potential travels continuously along the axons. • For example, in unmyelinated C fibers that conduct pain or temperature (0. 4– 1. 2 μm in diameter), conduction velocity along the axon is 0. 5– 2. 0 m/s (as fast as you walk or jog). • In contrast, among the myelinated nerve fibers, axons are mostly covered by myelin sheaths, and transmembrane currents can only occur at the nodes of Ranvier where the axonal membrane is exposed. • Myelin is rich in lipids (approximately 80%) and can therefore act as an insulator (meaning high transverse resistance and a low electrical capacitance) along the internodal segments. For example, conduction velocity in the most thoroughly myelinated axons (12– 20 μm in diameter) is 70– 120 m/s (race car speed), although other factors such as axon caliber can influence this velocity.

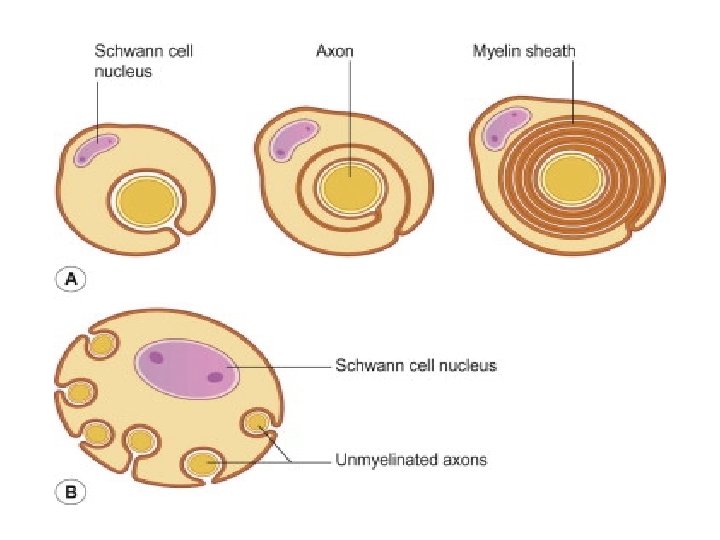
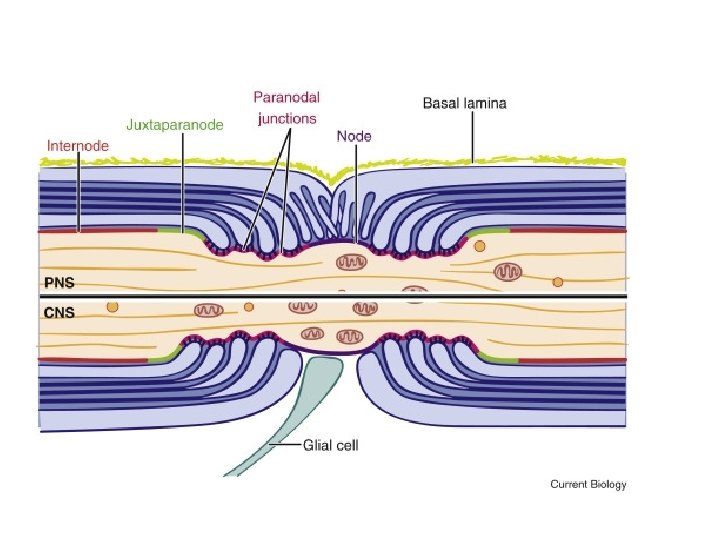
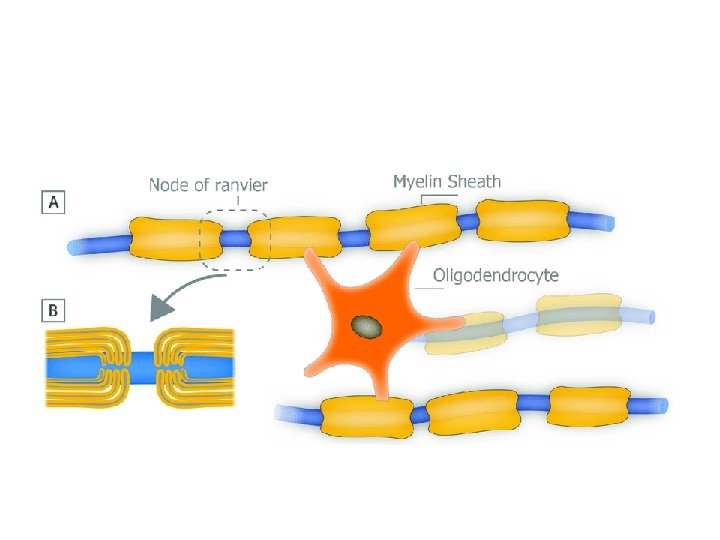
Are all axons covered with myelin? • No; they can be either myelinated or unmyelinated. Myelinated axons are ensheathed along their entire length. • The axon caliber (diameter) in mammalian PNS ranges from 0. 1 μm to 20 μm, with unmyelinated axons being less than 2 μm and myelinated axons being more than 1– 2 μm in diameter. • In the CNS, almost all axons with diameters greater than 0. 2 μm are myelinated. • In cross section, the myelinated axon appears as a nearly circular profile surrounded by a spirally wound multilamellar sheath. • Amazingly, a large myelinated axon may have up to 250 to 300 turns of myelin wrapping around it. • The ratio between axon diameter and that of the total nerve fiber (axon and myelin) is 0. 6– 0. 7, a ratio that is well maintained regardless of the axon caliber. • The length of the myelin sheath along the axon is approximately 1 mm in the PNS. Between two adjacent myelin segments, there approximately 1 -μm-long gaps called nodes of Ranvier. • At the nodes, the axon is exposed to the extracellular space.



• Myelin is considered a defining characteristic of the jawed vertebrates (gnathostomes), though axons are ensheathed by a type of cell, called glial cells, in invertebrates. • These glial wraps are quite different from vertebrate compact myelin, formed, as indicated above, by concentric wrapping of the myelinating cell process multiple times around the axon. • Myelin was first described in 1854 by Rudolf Virchow, although it was over a century later, following the development of electron microscopy, that its glial cell origin and its ultrastructure became apparent. • In vertebrates, not all axons are myelinated. For example, in the PNS, a large proportion of axons are unmyelinated. Instead, they are ensheathed by non-myelinating Schwann cells known as Remak SCs and arranged in Remak bundles. • In the CNS, non-myelinated axons (or intermittently myelinated axons, meaning axons with long non-myelinated regions between myelinated segments) intermingle with myelinated ones and are entwined, at least partially, by the processes of another type of glial cell called the astrocyte




MYELIN SHEATH • Much like the insulation around the wires in electrical systems, glial cells form a membraneous sheath surrounding axons called myelin, thereby insulating the axon. • This myelination, as it is called, can greatly increase the speed of signals transmitted between neurons (known as action potentials). Indeed, the evolution of myelin allowed vertebrates to achieve efficient nervous systems despite their large body size.


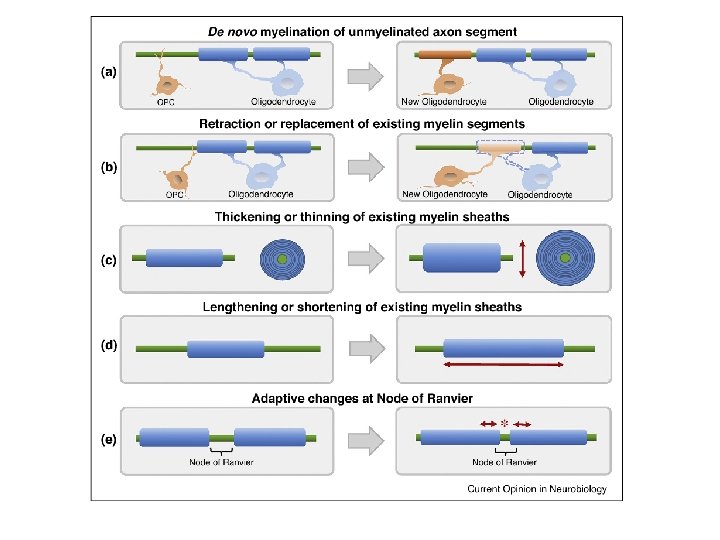
MYELINATION • The process of generating myelin is called myelination or myelinogenesis. • In the CNS, cells called oligodendrocyte precursor cells (OPCs; the precursors of oligodendrocytes) differentiate into mature oligodendrocytes, which form myelin. • In humans, myelination begins early in the 3 rd trimester, although only little myelin is present in either the CNS or the PNS at the time of birth. • During infancy, myelination progresses rapidly, with increasing numbers of axons acquiring myelin sheaths. • This corresponds with the development of cognitive and motor skills, including language comprehension, speech acquisition, crawling and walking. • Myelination continues through adolescence and early adulthood and although largely complete at this time, myelin sheaths can be added in grey matter regions such as the cerebral cortex, throughout life.


MYELIN COMPOSITION • CNS myelin differs slightly in composition and configuration from PNS myelin, but both perform the same "insulating" function. • Being rich in lipid, myelin appears white, hence the name given to the "white matter" of the CNS. • Both CNS white matter tracts (e. g. the optic nerve, corticospinal tract and corpus callosum) and PNS nerves (e. g. the sciatic nerve and the auditory nerve, which also appear white) each comprise thousands to millions of axons, largely aligned in parallel. • Blood vessels provide the route for oxygen and energy substrates such as glucose to reach these fibre tracts, which also contain other cell types including astrocytes and microglia in the CNS and macrophages in the PNS.

MYELIN COMPOSITION • In terms of total mass, myelin comprises approximately 40% water; the dry mass comprises between 60% and 75% lipid and between 15% and 25% protein. • Protein content includes myelin basic protein (MBP), which is abundant in the CNS where it plays a critical, non-redundant role in formation of compact myelin; myelin oligodendrocyte glycoprotein (MOG), which is specific to the CNS; and proteolipid protein (PLP), which is the most abundant protein in CNS myelin, but only a minor component of PNS myelin. • In the PNS, myelin protein zero (MPZ or P 0) has a similar role to that of PLP in the CNS in that it is involved in holding together the multiple concentric layers of glial cell membrane that constitute the myelin sheath. • The primary lipid of myelin is a glycolipid called galactocerebroside. The intertwining hydrocarbon chains of sphingomyelin strengthen the myelin sheath. Cholesterol is an essential lipid component of myelin, without which myelin fails to form.


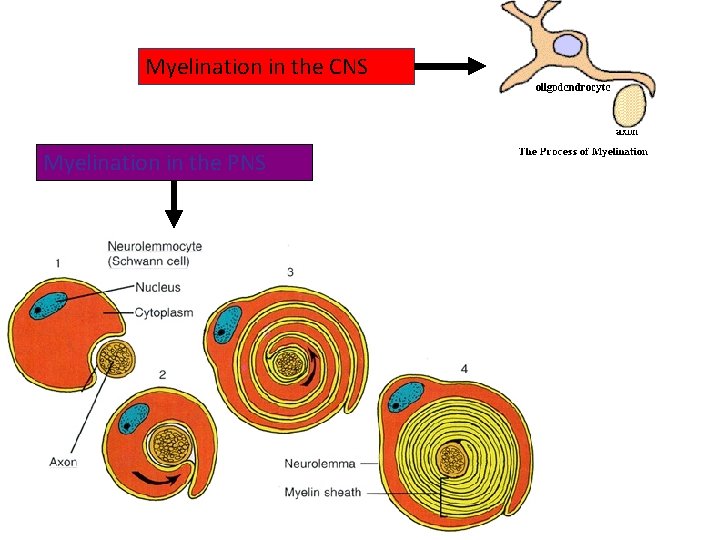
Myelination in the CNS Myelination in the PNS

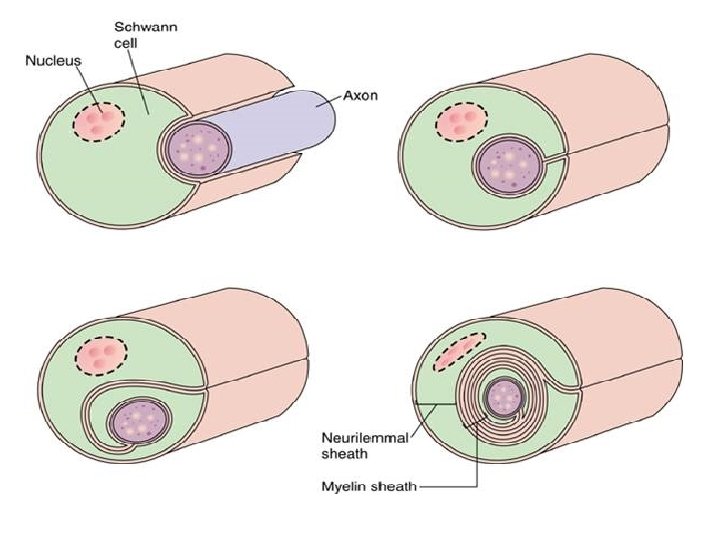
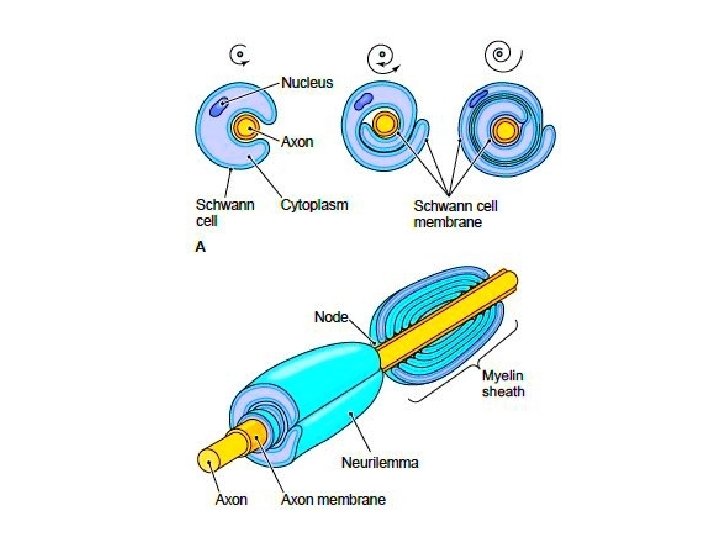
• Each Schwann Cell covers only a small segment of the Axon (0. 08 -0. 1 mm), and the entire myelin sheath is made up of membrane wrappings of successive Schwann Cells. • The junction where two successive schwann cells meet and where a small length of Axon is not covered by myelin is called the node of Ranvier. • The myelin is lipid-rich because as the Schwann cell winds around the axon, its cytoplasm is extruded from between the opposing layers of the plasma membrane. • However, the TEM shows small amounts of cytoplasm in several locations:

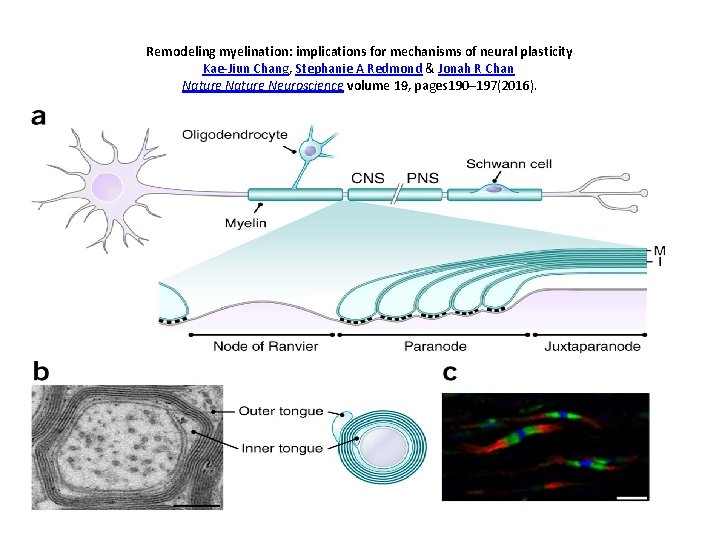
Remodeling myelination: implications for mechanisms of neural plasticity Kae-Jiun Chang, Stephanie A Redmond & Jonah R Chan Nature Neuroscience volume 19, pages 190– 197(2016).

• - the inner collar of Schwann cell cytoplasm, between axon and myelin • The Schmidt-Lanterman clefts, small islands within successive lamellae of myelin • Perinodal cytoplasm, at the node of Ranvier • Outer Collar of perinuclear cytoplasm around the myelin • If one conceptually unrolls the schwan cell its full extent can be apprecited • Also it can be seen that inner collar of schwan cell cytoplasmis continuous with the body of schwann cell through the Schmidt-Lanterman clefts anfd through the perinodal cytoplasm

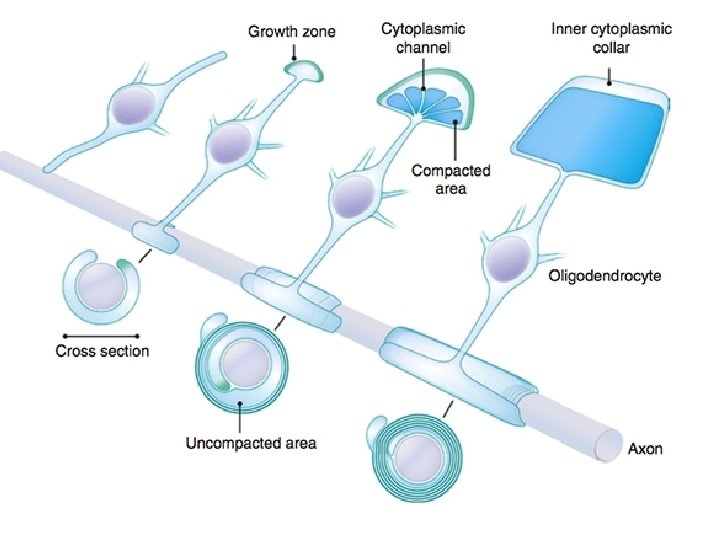
• Initially, a Schwann cell surrounds a length of nerve fibre and encloses it. When the axon is completely enclosed the outer surfaces of the membrane bounding the enveloping lips come into apposition, so that a double membrane, termed the mesaxon , is formed. • The mesaxon then elongates and forms a loose spiral around the enclosed axon. • At this stage, Schwann cell cytoplasm is present between each of the turns of the mesaxon, but as the mesaxon elongates further the cytoplasm is eliminated so that the cytoplasmic surfaces of the membranes of adjacent turns come into contact, and a thick or maior dense line is formed by this apposition. • Compact myelin results; and the major dense line, which separates the lamellae, alternates with the intraperiod line which is formed within the mesaxon where the external surfaces of the membrane come together.


• In their electron microscope studies on osmiumfixed material, Luse using young rats and mice and De Robertis, Gerschenfeld, and Wald using young cats and rats, suggest that central myelin is formed in a different manner to peripheral myelin. • Luse concludes that within a length of central myelin the lamellae are not produced by the elongation of a single membrane as within an internode in the peripheral nervous system, but by the plication of the membranes of several glial cells, and, in particular, those of the oligodendrocytes. • Her evidence is inconclusive and contrary to that of De Robertis, Gerschenfeld, and Wald , who, although they consider that the oligodendrocytes form the myelin, put forward the hypothesis that the lamellae arise within the cytoplasm of these cells, which have a well developed endoplasmic reticulum.

• These theories imply the absence of both a spiral and an internal mesaxon, although De Robertis, Gerschenfeld, and Wald find that internal mesaxons are occasionally present. • In an earlier electron microscope study based on potassium permanganate fixed optic nerves and spinal cords from tadpoles of the toad Xenopus laevis Daudin, the present author presented evidence against the above theories. • In these tadpoles the myelin lamellae arranged in a spiral and internal mesaxons are always present. • Further, no discontinuities in the myelin lamellae have been observed, though these would be expected, at least during the formation of the lamellae, if the mechanisms suggested by either Luse or De Robertis, Gershenfeld, and Wald are correct.


• In central myelin, tile outer cytoplasm is confined to a tongue, which varies in size, so that in a very few cases it may be as extensive as that of peripheral nerves, when an external mesaxon is present. • However, the tongue is usually small and because cytoplasm is absent over most of the outer surface of the myelin, adjacent sheaths come into contact. • Such contacts have not been recorded in the peripheral nervous system where the outer layer of cytoplasm always separates the sheaths The process by which myelin is formed in the central nervous system, is similar to that described in the peripheral nervous system although minor differences occur. • In both cases, the first step in myelinogenesis is one in which the nerve fibre is surrounded by the myelin-forming cell. In the peripheral nervous system each Schwann cell may surround a number of nerve fibres initially, so that in a section of a developing nerve, a Schwann cell may be seen at the centre of a group of nerve fibres, each of which is embedded in its cytoplasm. • However, before a mesaxon is formed the number of Schwann cells increases, so that each contains only one myelinating nerve fibre. • In the central nervous system the arrangement is somewhat different, for at any stage of myelinogenesis never more than one nerve fibre has been found embedded in a myelin-forming cell process

• However no nuclei have been located in the tongues within the immediate vicinity of the sheath, as they are in the peripheral nervous system, so that the disposition of the myelin-forming cells must certainly be different from that of the peripheral nervous system where the nuclei of the Schwann cells are encountered within the outer cytoplasm. • In the optic nerve of the rat, for example, the glial cells are arranged in columns between which run bundles of nerve fibres completely devoid of nuclei. • Therefore on structural grounds alone it is probable that the nucleuscontaining body of the myelin-forming cell is some distance from the site where the myelin is formed. • This arrangement could account for both the absence of nuclei within the tongues as well as the failure to find connections between tongues and glial cells, since thin sections so essential to electron microscopy would tend to preclude the possibility of obtaining the whole length of a connection in one section.

• The cytoplasm of the myelin-forming processes is akin to that of the oligodendrocytes, but differs from the astrocyte cytoplasm, which suggests that the former may be responsible for the formation of myelin. • In addition, the oligodendrocytcs have a well developed endoplasmic reticulum, which is consistent with an active metabolism, such as would occur during the synthesis of cell membranes necessary for the formation of myelin. • Both astrocytes and oligodendrocytes have both been held responsible and the formation of myelin has even been attributed to the axons themselves.

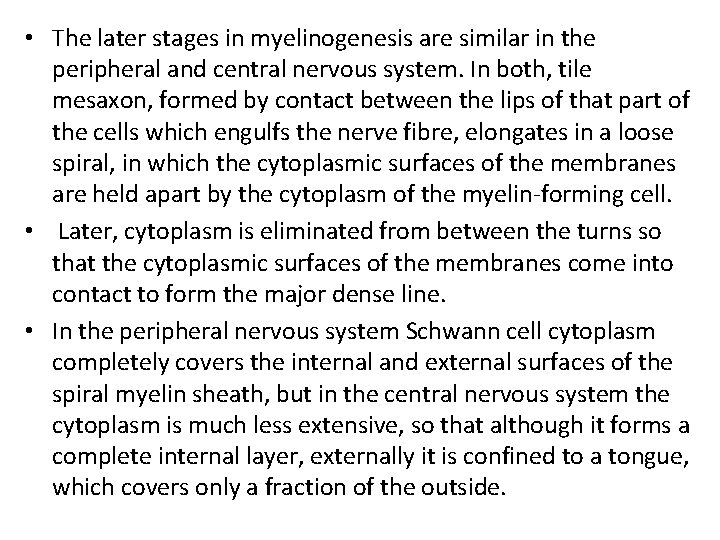
• The later stages in myelinogenesis are similar in the peripheral and central nervous system. In both, tile mesaxon, formed by contact between the lips of that part of the cells which engulfs the nerve fibre, elongates in a loose spiral, in which the cytoplasmic surfaces of the membranes are held apart by the cytoplasm of the myelin-forming cell. • Later, cytoplasm is eliminated from between the turns so that the cytoplasmic surfaces of the membranes come into contact to form the major dense line. • In the peripheral nervous system Schwann cell cytoplasm completely covers the internal and external surfaces of the spiral myelin sheath, but in the central nervous system the cytoplasm is much less extensive, so that although it forms a complete internal layer, externally it is confined to a tongue, which covers only a fraction of the outside.


• This structure of central sheaths which was first described in Xenopus tadpoles has in the present study been confirmed in the optic nerve of the rat. • The structure of the nodes of Ranvier in the peripheral nervous system has been described by Uzman and Nogueira-Graf Robertson and Engstrom and Wersall However, there has been no previous information about the structure of central nodes, although on the basis of light microscopy their presence has been recorded by a number of workers. • In both cases the sheath terminates as the spiralled lamellae turn off from the inside to enclose a helix of cytoplasm derived from the myelin-forming cell.


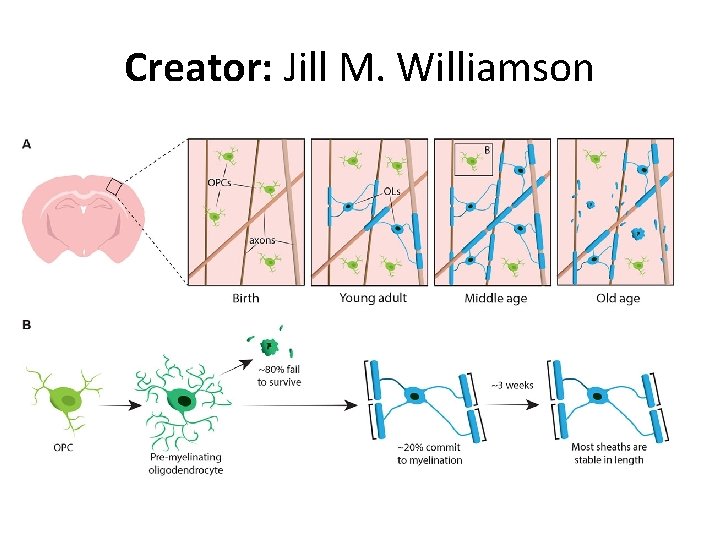
Creator: Jill M. Williamson


• One of the most significant paradigm shifts in membrane remodeling is the emerging view that membrane transformation is not exclusively controlled by cytoskeletal rearrangement, but also by biophysical constraints, adhesive forces, membrane curvature and compaction. • One of the most exquisite examples of membrane remodeling is myelination. The advent of myelin was instrumental in advancing the nervous system during vertebrate evolution. • With more rapid and efficient communication between neurons, faster and more complex computations could be performed in a given time and space

Axonal Signaling Regulates Myelination • • • How is the spiral wrapping of the myelin sheath around axons formed precisely and appropriately? One mechanism has been identified in PNS myelination. In the PNS, neuregulin 1 type III protein is expressed on the axon surface and interacts with glial Erb. B receptors, and it has a pivotal role for Schwann cell differentiation and myelination (for review, see Nave & Salzer 2006). Unmyelinated autonomic neurons express low levels of neuregulin 1 type III on the axon surface, whereas heavily myelinated axons express high levels. Without neuregulin 1 type III, Schwann cells in culture derived from these mutant mice cannot myelinate neurons in the spinal cord (dorsal root ganglion neurons). Intriguingly, in normally unmyelinated fibers, forced expression of neuregulin 1 type III in the postganglionic fibers of sympathetic neurons grown in culture can be forced to myelinate. Thus, the level of neuregulin 1 type III on the PNS axons is a key instructive signal for myelination. Furthermore, above threshold, the myelin formation is correlated with the amount of neuregulin 1 type III presented by the axon to the Schwann cell. Reduced expression of neuregulin 1 type III leads to a thinner than normal myelin sheath in the heterozygous mutant mice of this molecule. In contrast, transgenic mice that overexpress neuregulin 1 become hypermyelinated. One interesting question is: Does neuregulin-Erb. B signaling regulate CNS myelination as well? Although several reports show that oligodendrocytes respond to neuregulin 1 in vitro, analyses of a series of conditional null mutant animals lacking neuregulin 1 showed normal myelination (Brinkmann et al. 2008). It is still unclear how myelination is regulated in the CNS.

From PNAS

• At nodes, voltage-gated sodium channels are highly accumulated and are responsible for the generation of action potentials. • To induce and maintain nodal sodium channel clusters, specific molecules are also enriched at nodal axons, including cell adhesion molecules such as neurofascin 186 and cytoskeletal and scaffolding proteins such as b. IV spectrin (Poliak & Peles 2003; Susuki & Rasband 2008). • The myelin helps assemble this nodal molecular organization. For example, during the development of PNS myelinated nerve fibers, a molecule called gliomedin is secreted from myelinating Schwann cells then incorporated into the extracellular matrix surrounding nodes, where it promotes assembly of nodal axonal molecules. • Due to the presence of the insulating myelin sheath at internodes and voltage-gated sodium channels at nodes, the action potential in myelinated nerve fibers jumps from one node to the next. • This mode of travel by the action potential is called "saltatory conduction" and allows for rapid impulse propagation.

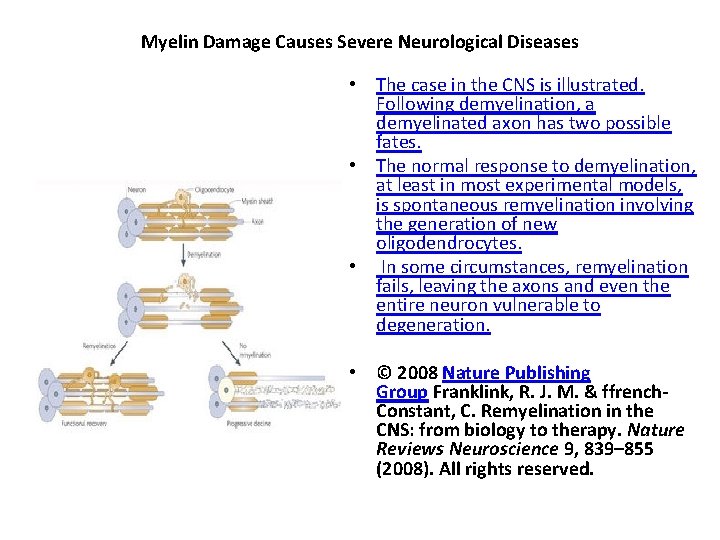
What happens if myelin is damaged? • The importance of myelin is underscored by the presence of various diseases in which the primary problem is defective myelination. • Demyelination is the condition in which preexisting myelin sheaths are damaged and subsequently lost, and it is one of the leading causes of neurological disease. • Primary demyelination can be induced by several mechanisms, including inflammatory or metabolic causes. • Myelin defects also occur by genetic abnormalities that affect glial cells. • Regardless of its cause, myelin loss causes remarkable nerve dysfunction because nerve conduction can be slowed or blocked, resulting in the damaged information networks between the brain and the body or within the brain itself. • Following demyelination, the naked axon can be re-covered by new myelin. • This process is called remyelination and is associated with functional recovery (Franklin and ffrench-Constant 2008). • The myelin sheaths generated during remyelination are typically thinner and shorter than those generated during developmental myelination. • In some circumstances, however, remyelination fails, leaving axons and even the entire neuron vulnerable to degeneration. • Thus, patients with demyelinating diseases suffer from various neurological symptoms.

Myelin Damage Causes Severe Neurological Diseases • The case in the CNS is illustrated. Following demyelination, a demyelinated axon has two possible fates. • The normal response to demyelination, at least in most experimental models, is spontaneous remyelination involving the generation of new oligodendrocytes. • In some circumstances, remyelination fails, leaving the axons and even the entire neuron vulnerable to degeneration. • © 2008 Nature Publishing Group Franklink, R. J. M. & ffrench. Constant, C. Remyelination in the CNS: from biology to therapy. Nature Reviews Neuroscience 9, 839– 855 (2008). All rights reserved.

MULTIPLE SCLEROSIS • The representative demyelinating disease, and perhaps the most well known, is multiple sclerosis (MS). • This autoimmune neurological disorder is caused by the spreading of demyelinating CNS lesions in the entire brain and over time (Siffrin et al. 2010). • Patients with MS develop various symptoms, including visual loss, cognitive dysfunction, motor weakness, and pain. • Approximately 80 percent of patients experience relapse and remitting episodes of neurologic deficits in the early phase of the disease (relapse-remitting MS). There are no clinical deteriorations between two episodes. • Approximately ten years after disease onset, about one-half of MS patients suffer from progressive neurological deterioration (secondary progressive MS). • Importantly, the loss of axons and their neurons is a major factor determining long-term disability in patients, although the primary cause of the disease is demyelination. • Several immunodulative therapies are in use to prevent new attacks; however, there is no known cure for MS.

• Thank-You