My Stem Autograft Preparation Kit Regenerative Treatments made







- Slides: 16

My. Stem ® Autograft Preparation Kit Regenerative Treatments made easy

The People Pier Ivona, IFATS Member, WOSIAM Member, Regenerative Medicine Expert, Entrepreneur Mr. Ivona has a multi year experience in Regenerative treatments and Med. Dev industry, His experience leads the team who developed My. Stem technology

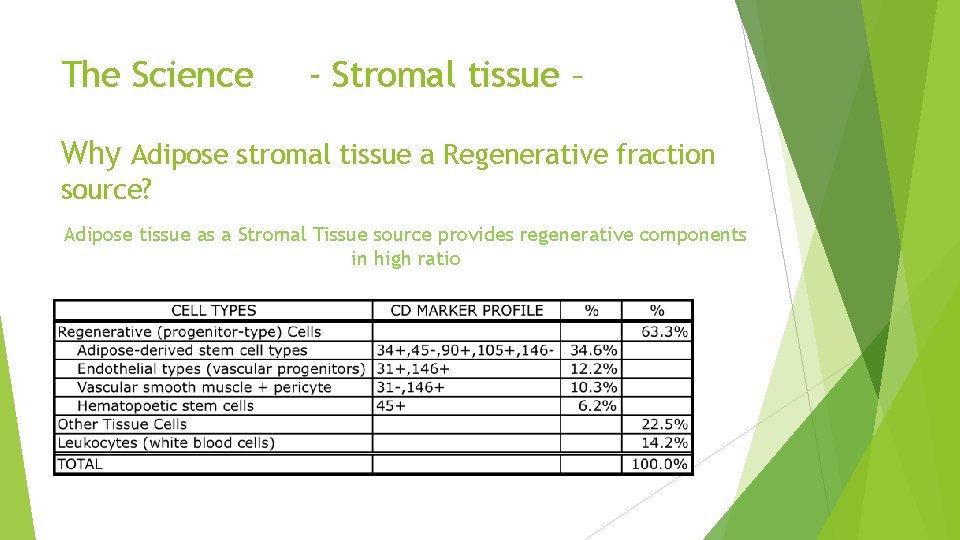
The Science - Stromal tissue Scientific Definition: The connective tissue framework of an organ, a gland, or other structure, as distinguished from the tissues performing the special function of the organ or part. Stroma is a broadly used term for the loose connective tissue that contains mesenchymal stem cells and other cells like fibroblasts, macrophages, adipocytes, mast cells and leukocytes. Source: The American Heritage® Stedman's Medical Dictionary

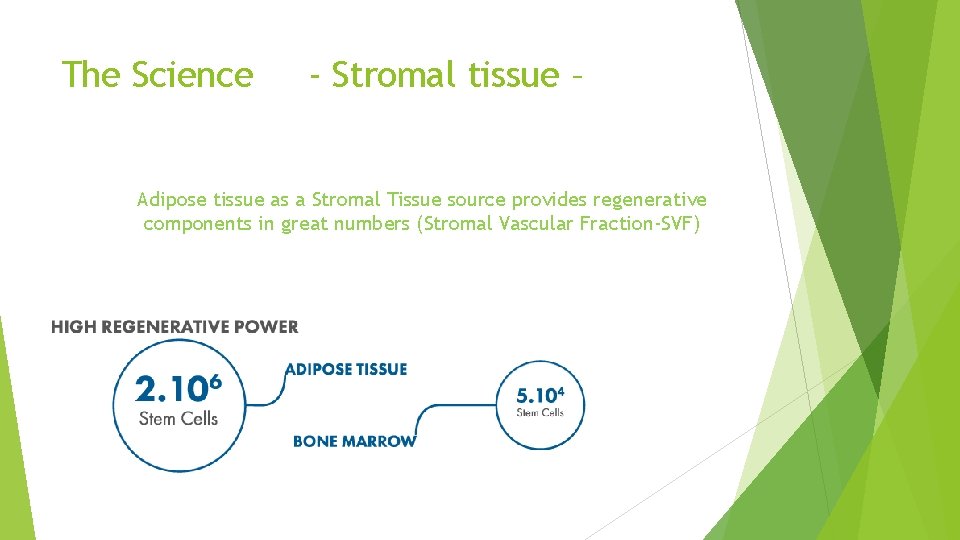
The Science - Stromal tissue – Why Adipose stromal tissue a Regenerative fraction source? Adipose tissue as a Stromal Tissue source provides regenerative components in high ratio

The Science - Stromal tissue – Adipose tissue as a Stromal Tissue source provides regenerative components in great numbers (Stromal Vascular Fraction-SVF)

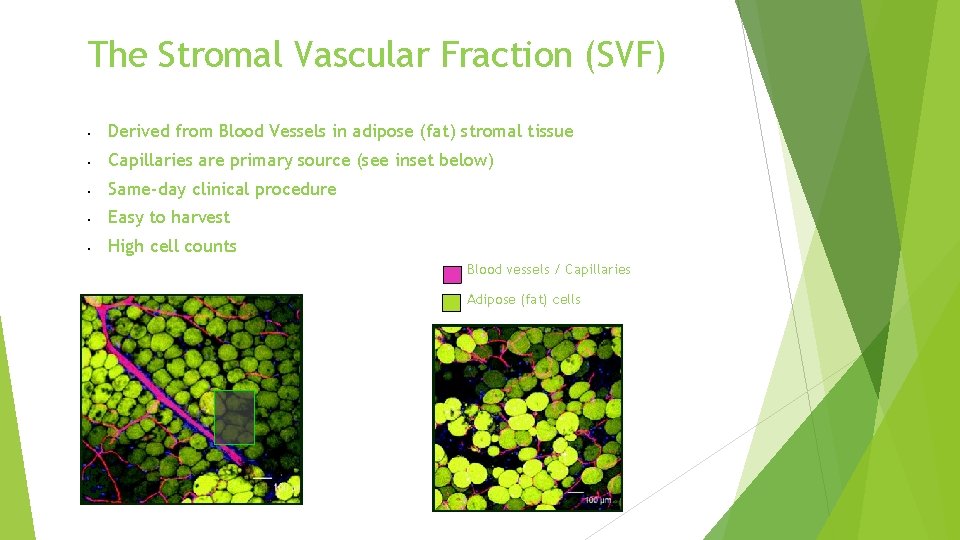
The Stromal Vascular Fraction (SVF) Derived from Blood Vessels in adipose (fat) stromal tissue Capillaries are primary source (see inset below) Same-day clinical procedure Easy to harvest High cell counts Blood vessels / Capillaries Adipose (fat) cells

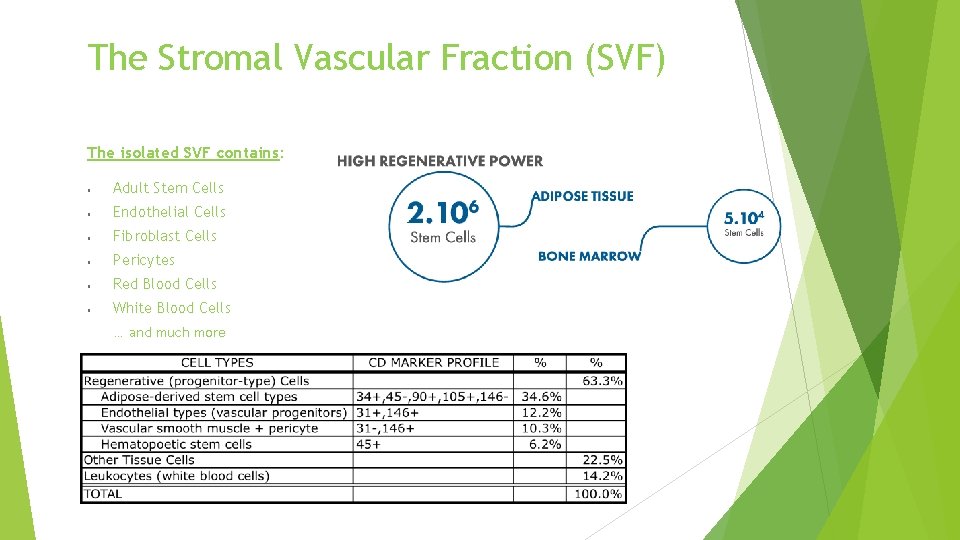
The Stromal Vascular Fraction (SVF) The isolated SVF contains: Adult Stem Cells Endothelial Cells Fibroblast Cells Pericytes Red Blood Cells White Blood Cells … and much more

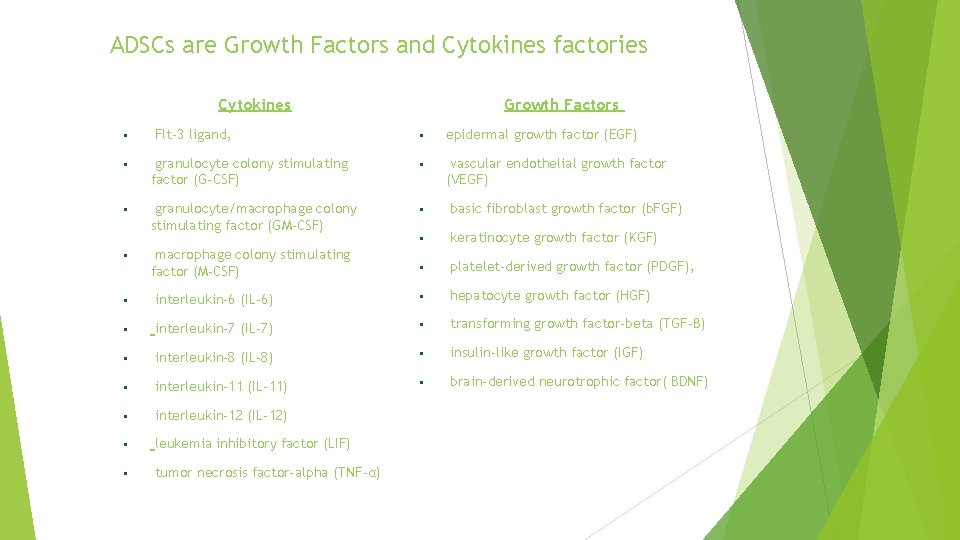
ADSCs are Growth Factors and Cytokines factories Cytokines Flt-3 ligand, Growth Factors epidermal growth factor (EGF) vascular endothelial growth factor (VEGF) granulocyte colony stimulating factor (G-CSF) granulocyte/macrophage colony stimulating factor (GM-CSF) macrophage colony stimulating factor (M-CSF) basic fibroblast growth factor (b. FGF) keratinocyte growth factor (KGF) platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF) interleukin-6 (IL-6) interleukin-7 (IL-7) transforming growth factor-beta (TGF-β) interleukin-8 (IL-8) insulin-like growth factor (IGF) interleukin-11 (IL-11) interleukin-12 (IL-12) leukemia inhibitory factor (LIF) tumor necrosis factor-alpha (TNF-α) brain-derived neurotrophic factor( BDNF)

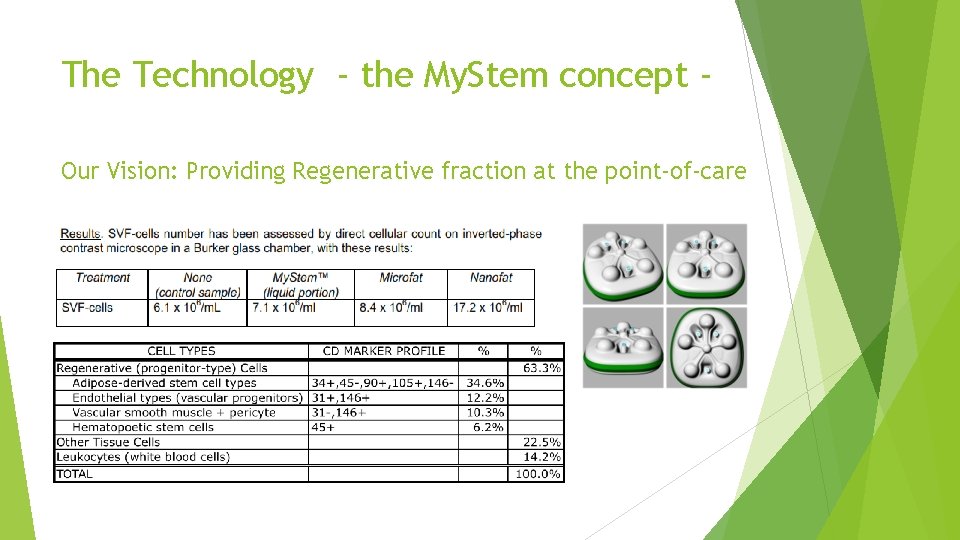
The Technology - the My. Stem concept Our Vision: Providing Regenerative fraction at the point-of-care

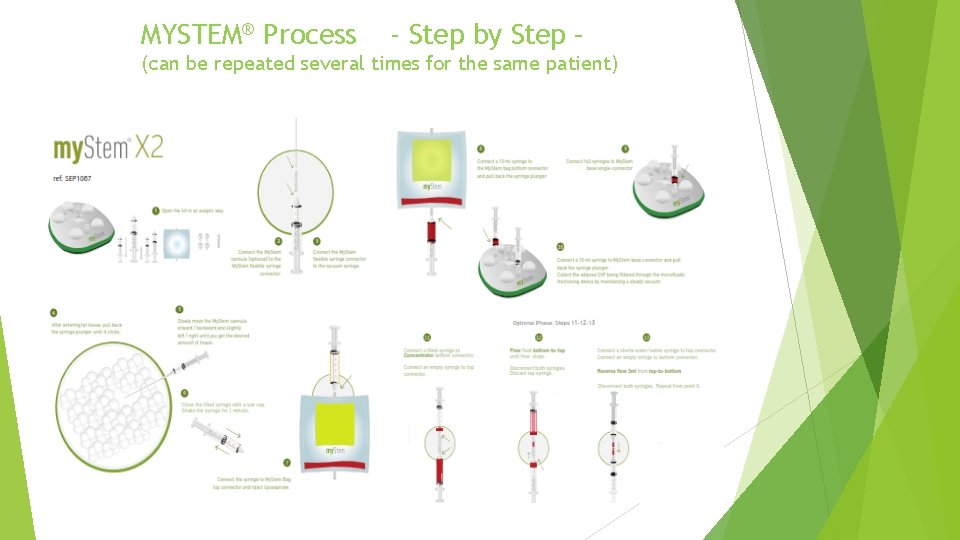
MYSTEM® Process - Step by Step – (can be repeated several times for the same patient)

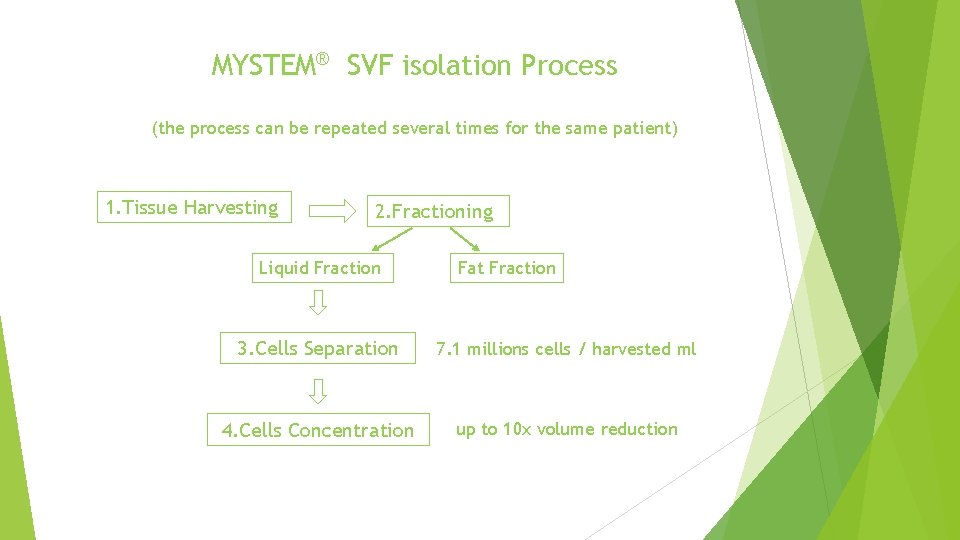
MYSTEM® SVF isolation Process (the process can be repeated several times for the same patient) 1. Tissue Harvesting 2. Fractioning Liquid Fraction Fat Fraction 3. Cells Separation 7. 1 millions cells / harvested ml 4. Cells Concentration up to 10 x volume reduction

The Technology - the My. Stem device • Single-use Medical Device • One-step Procedure • Process time 15 min • 710. 000 Nucleated Cells / ml • Up to 10 x Concentration • International Patent

Clinical use of My. Stem technology Demonstrated effects in: Bone tissue repair (Published Study) Osteoarthritis (Case Report) Intervertebral Disc disease (Published Study) Tendon injury (Case Report) Severe Burns (Case Report) Soft tissue augmentation (Case Report) Wound healing (Case Report) Diabetic Foot Syndrome (Case Report) Crohns (Case Report) Fistulas (Case Report)

My. Stem Factsheet My. Stem Authorizations • CE Mark • SFDA (Saudi FDA) • TGA (Australia) • ANVISA (Brazil) Clinical Evidence over 1200 clinical cases done

Together. . . . helping people. . . with great results. .

Thank you
 Autograft example
Autograft example Digital inverter
Digital inverter Regenerative nodules of liver
Regenerative nodules of liver Advantages and disadvantages of plugging braking
Advantages and disadvantages of plugging braking Documentaire francais
Documentaire francais Mrp process
Mrp process Regenerative type heat exchanger
Regenerative type heat exchanger Anemie arégénérative
Anemie arégénérative Vertical shell and tube heat exchanger
Vertical shell and tube heat exchanger Regenerative medicine
Regenerative medicine Aloe vera with mosqueta rose oil regenerative
Aloe vera with mosqueta rose oil regenerative Regenerative type heat exchanger
Regenerative type heat exchanger Hiv treatments
Hiv treatments Multi-element design
Multi-element design Dementia treatments and interventions near patterson
Dementia treatments and interventions near patterson Diabetes treatments
Diabetes treatments Treatments for acute renal failure
Treatments for acute renal failure