Mutation Analysis using Temperature Gradient Gel Electrophoresis Overview





- Slides: 36

Mutation Analysis using Temperature Gradient Gel Electrophoresis

Overview 1. 2. 3. 4. 5. Introduction Basic principle of TGGE Applications Optimizing new assays Versatility of TGGE systems

Introduction q q TGGE is a type of acrylamide gel electrophoresis which is used to detect point mutations and polymorphisms within PCR-products. TGGE is very fast and sensitive in detecting heterozygous sequence variations within the PCRproduct. These qualities make TGGE the screening method of choice

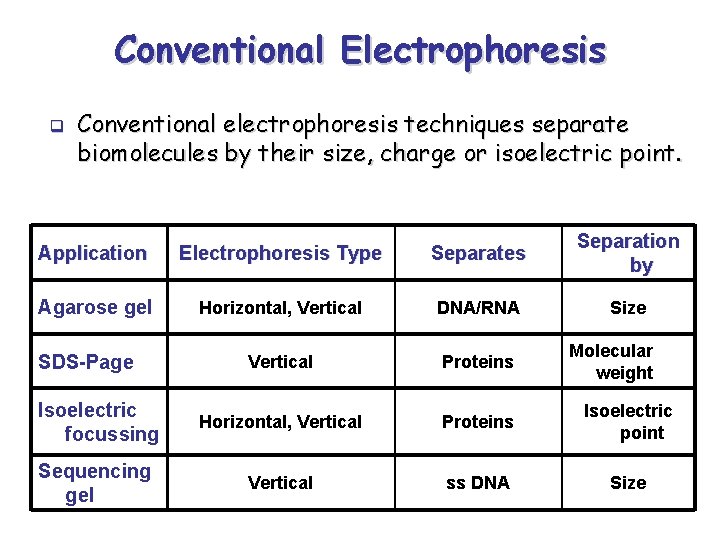
Conventional Electrophoresis q Conventional electrophoresis techniques separate biomolecules by their size, charge or isoelectric point. Application Electrophoresis Type Separates Separation by Agarose gel Horizontal, Vertical DNA/RNA Size Vertical Proteins Isoelectric focussing Horizontal, Vertical Proteins Isoelectric point Sequencing gel Vertical ss DNA Size SDS-Page Molecular weight

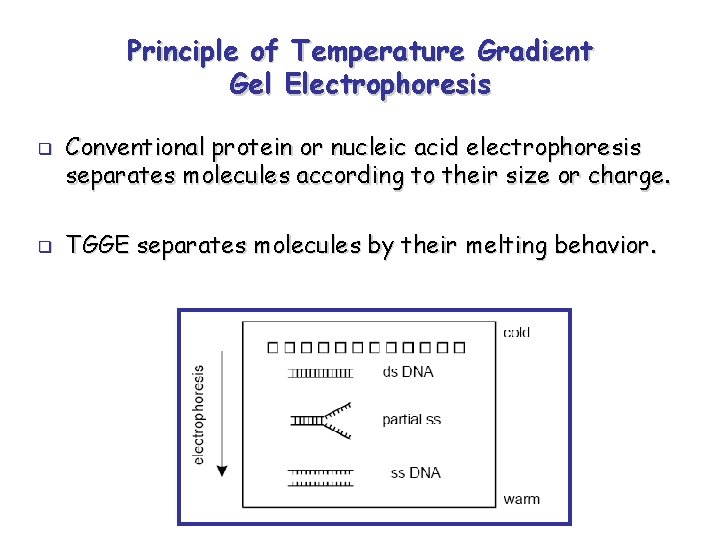
Principle of Temperature Gradient Gel Electrophoresis q q Conventional protein or nucleic acid electrophoresis separates molecules according to their size or charge. TGGE separates molecules by their melting behavior.

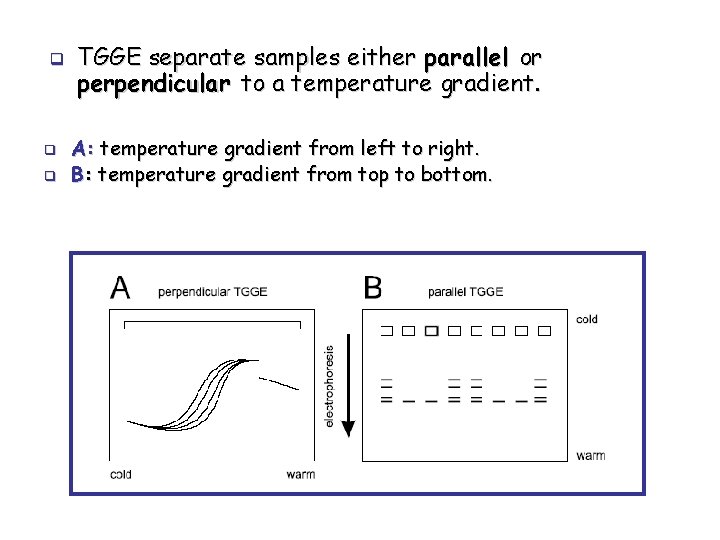
q q q TGGE separate samples either parallel or perpendicular to a temperature gradient. A: temperature gradient from left to right. B: temperature gradient from top to bottom.

Application of TGGE Two major filed of application for TGGE: q Mutation Analysis in PCR Fragment. q Diversity Analysis of Complex Bacterial samples.

Establishing TGGE protocols … q Design of PCR fragment. q In silico optimization q Finding the optimal temperature gradient q Transfer from perpendicular to parallel TGGE for routine analyses

Design of PCR fragment Gene fragment of interest q PCR primers should be designed with a conventional computer program. q The melting behavior of the resulting fragment should then be checked with the Poland software. q It is essential that the DNA fragment shows different melting domains. q If there is only one single melting domain, an artificial higher melting domain (called GC clamp) must be added during PCR.

In silico optimization q Computer analysis can be done prior to starting electrophoresis. Poland analysis q q The Poland software calculates the melting behavior of a DNA fragment according to its base sequence. The ideal fragment shows at least two distinct melting domains, mutation can only be detected in the lower melting domain.

TGGE flow chart of success q Step I II III (http: //www. biophys. uni-duesseldorf. de/POLAND/poland. html). CGGGGGCGGCGGGCGCGGGGCGCGGCGACATCT GGACCCAACTCCTG

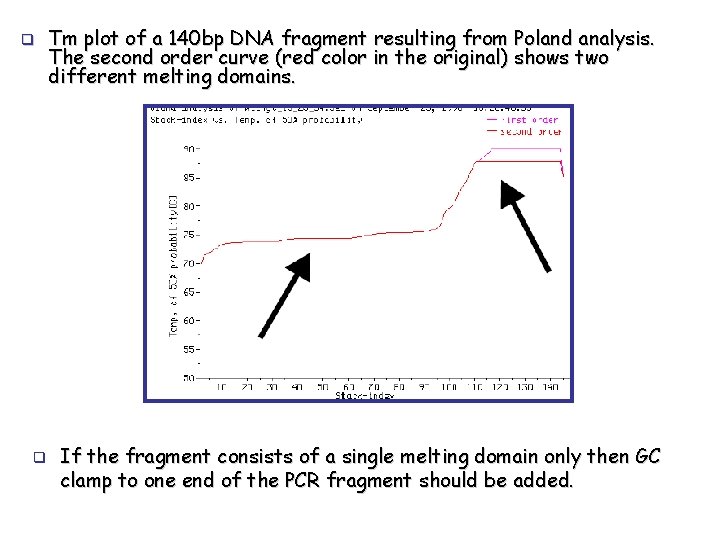
q q Tm plot of a 140 bp DNA fragment resulting from Poland analysis. The second order curve (red color in the original) shows two different melting domains. If the fragment consists of a single melting domain only then GC clamp to one end of the PCR fragment should be added.

GC clamps q q GC clamp is an artificial, high melting domain which is attached to one end of the fragment during PCR. The name “GC clamp” clamp implies that this short stretch will hold the DNA fragment together, Prevent dissociation into the single strands at higher temperatures

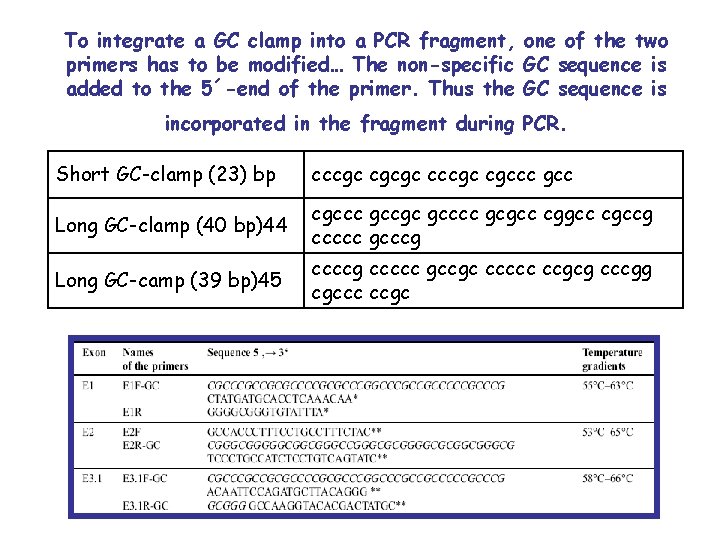
To integrate a GC clamp into a PCR fragment, one of the two primers has to be modified… The non-specific GC sequence is added to the 5´-end of the primer. Thus the GC sequence is incorporated in the fragment during PCR. Short GC-clamp (23) bp cccgc cgcgc cccgc cgccc gcc Long GC-clamp (40 bp)44 cgccc gccgc gcccc gcgccg ccccc gcccg Long GC-camp (39 bp)45 ccccg ccccc gccgc ccccc ccgcg cccgg cgccc ccgc

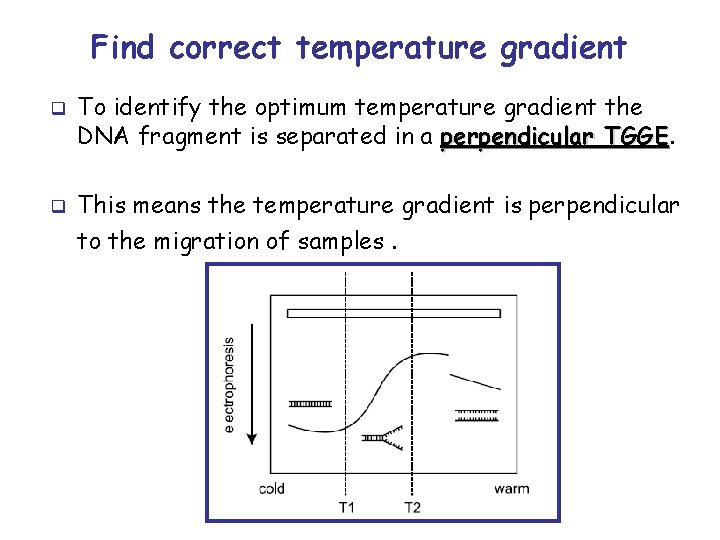
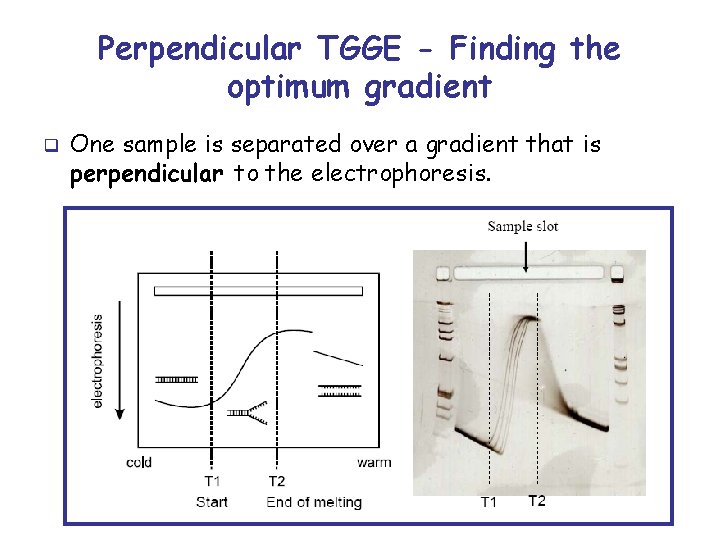
Find correct temperature gradient q q To identify the optimum temperature gradient the DNA fragment is separated in a perpendicular TGGE This means the temperature gradient is perpendicular to the migration of samples.

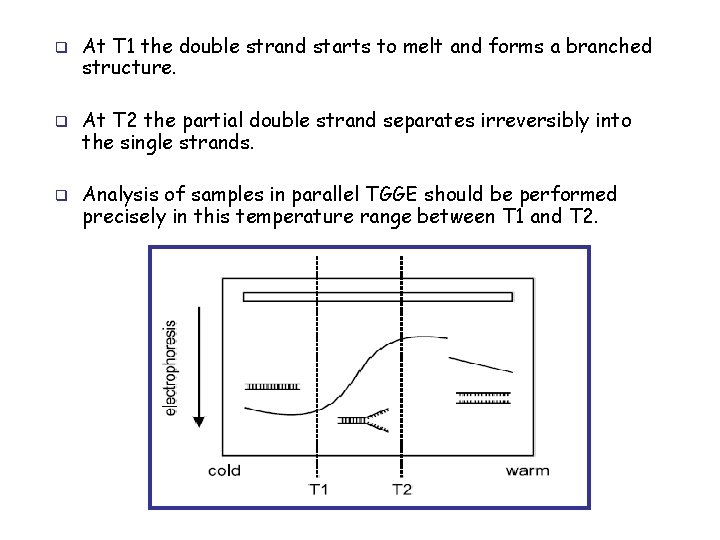
q q q At T 1 the double strand starts to melt and forms a branched structure. At T 2 the partial double strand separates irreversibly into the single strands. Analysis of samples in parallel TGGE should be performed precisely in this temperature range between T 1 and T 2.

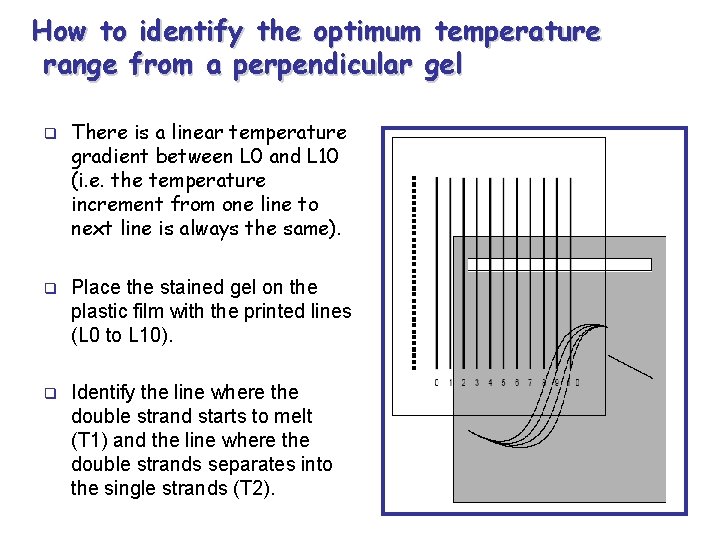
How to identify the optimum temperature range from a perpendicular gel q There is a linear temperature gradient between L 0 and L 10 (i. e. the temperature increment from one line to next line is always the same). q Place the stained gel on the plastic film with the printed lines (L 0 to L 10). q Identify the line where the double strand starts to melt (T 1) and the line where the double strands separates into the single strands (T 2).

Perpendicular TGGE - Finding the optimum gradient q One sample is separated over a gradient that is perpendicular to the electrophoresis.

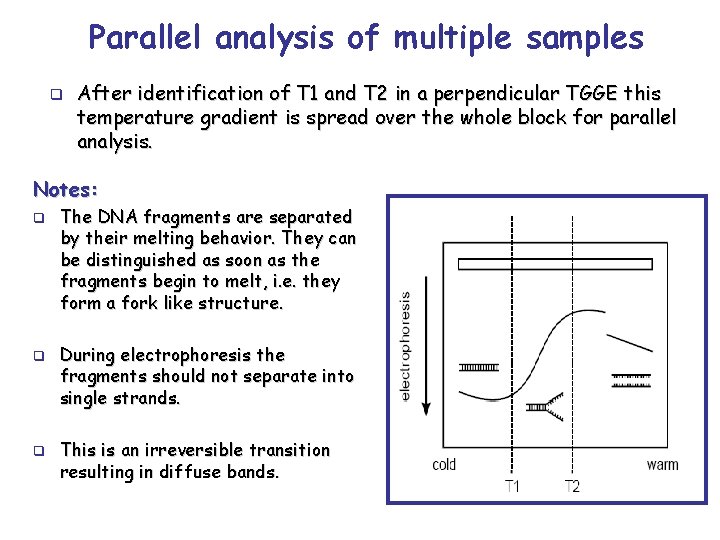
Parallel analysis of multiple samples q After identification of T 1 and T 2 in a perpendicular TGGE this temperature gradient is spread over the whole block for parallel analysis. Notes: q q q The DNA fragments are separated by their melting behavior. They can be distinguished as soon as the fragments begin to melt, i. e. they form a fork like structure. During electrophoresis the fragments should not separate into single strands. This is an irreversible transition resulting in diffuse bands.

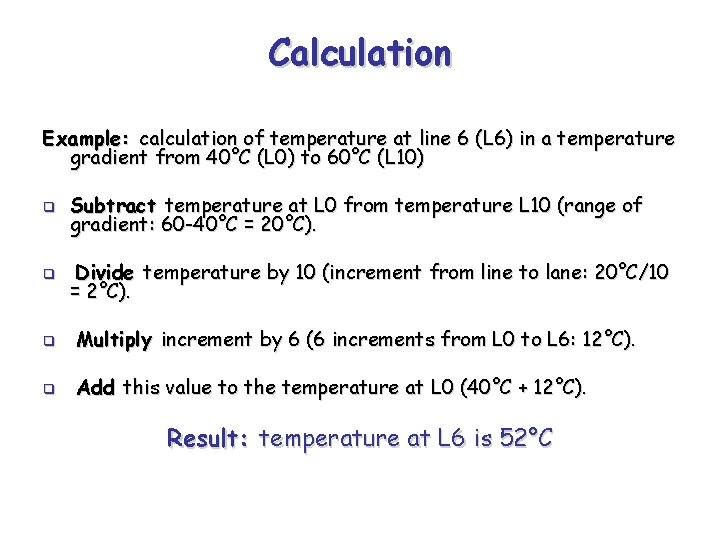
Calculation Example: calculation of temperature at line 6 (L 6) in a temperature gradient from 40°C (L 0) to 60°C (L 10) q Subtract temperature at L 0 from temperature L 10 (range of gradient: 60 -40°C = 20°C). q Divide temperature by 10 (increment from line to lane: 20°C/10 = 2°C). q Multiply increment by 6 (6 increments from L 0 to L 6: 12°C). q Add this value to the temperature at L 0 (40°C + 12°C). Result: temperature at L 6 is 52°C

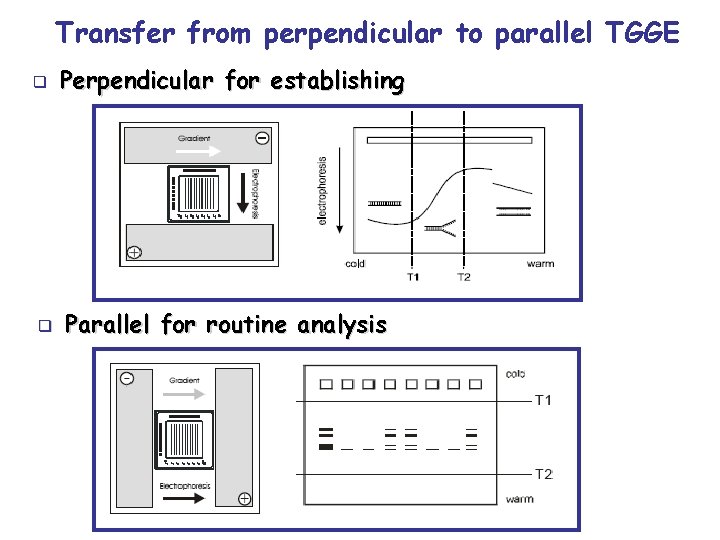
Transfer from perpendicular to parallel TGGE q q Perpendicular for establishing Parallel for routine analysis

Tips from the bench q In contrast to conventional electrophoresis, in TGGE the migration length does NOT only depend on running time, but rather on the temperature gradient. Extended running times do not necessarily lead to a better separation of bands Instead, the temperature gradient should be optimized … !

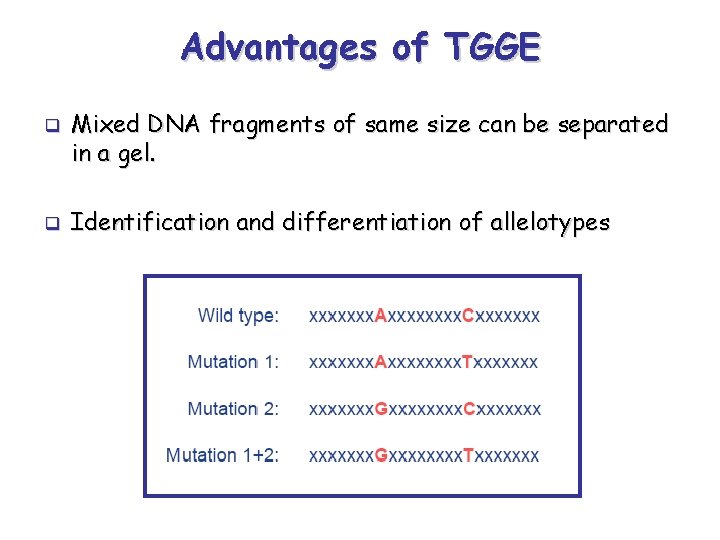
Advantages of TGGE q q Mixed DNA fragments of same size can be separated in a gel. Identification and differentiation of allelotypes

Versatility of TGGE systems q TGGE systems could be also used for : q DGGE (Denaturing Gradient Gel Electrophoresis) q CDGE (Constant Denaturing Gel Electrophoresis) q SSCP (Single Strand Conformation Polymorphism)

Versatility of TGGE systems q DNA based TGGE applications : q Mutation analyses q Heteroduplex analyses (HA) q Imprinting studies (= DNA methylation) q Differentiation of amplicon and competitor for quantitative DNA analyses

Versatility of TGGE systems q q RNA based TGGE application : q Secondary structure analysis of RNA q Analysis of ds. RNA molecules Protein based TGGE applications : q Thermal stability analysis of proteins q Protein/protein or protein/ligand interaction analysis

TGGE Systems TGGE System Small separation distance q Short running times q TGGE Maxi System Long separation distance q Ideal for complex mixtures q High parallel sample throughput q

The TGGE system System overview The TGGE system consists of three components: 1) Electrophoresis unit : including thermoblock, buffer chambers, safety lid. 2) Controller: control of electrophoresis parameters (Voltage) and temperature gradient. 3) Power supply : power supply for electrophoresis unit and controller.

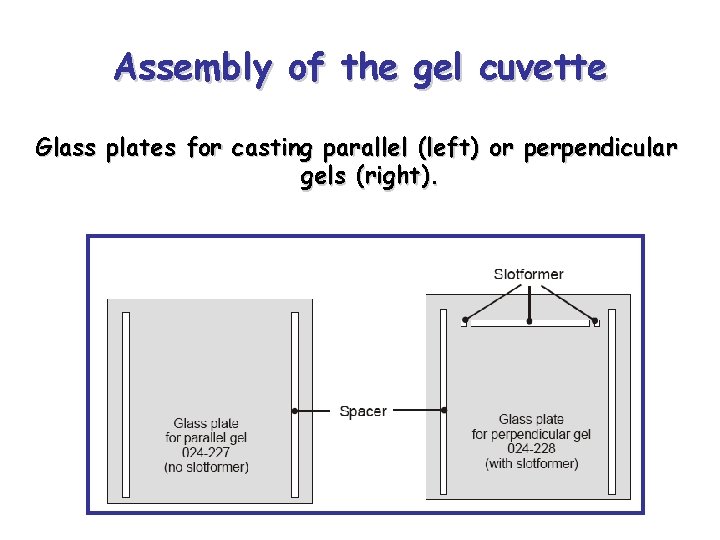
Assembly of the gel cuvette Glass plates for casting parallel (left) or perpendicular gels (right).

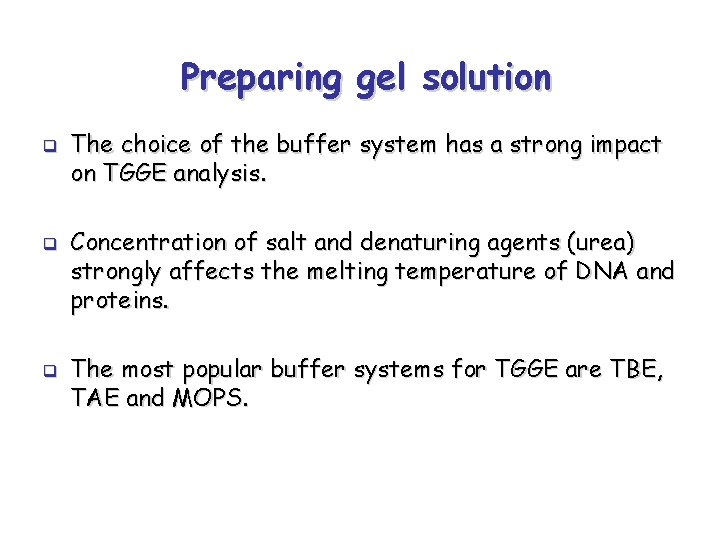
Preparing gel solution q q q The choice of the buffer system has a strong impact on TGGE analysis. Concentration of salt and denaturing agents (urea) strongly affects the melting temperature of DNA and proteins. The most popular buffer systems for TGGE are TBE, TAE and MOPS.

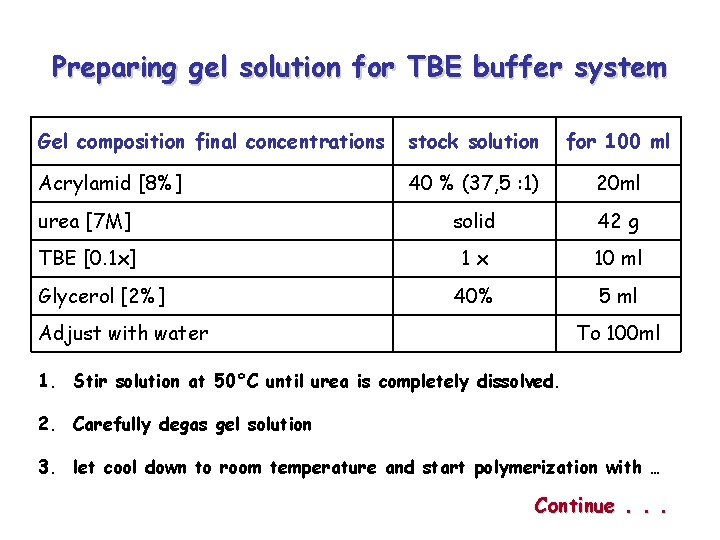
Preparing gel solution for TBE buffer system Gel composition final concentrations stock solution for 100 ml Acrylamid [8%] 40 % (37, 5 : 1) 20 ml urea [7 M] solid 42 g TBE [0. 1 x] 1 x 10 ml 40% 5 ml To 100 ml Glycerol [2%] Adjust with water 1. Stir solution at 50°C until urea is completely dissolved. 2. Carefully degas gel solution 3. let cool down to room temperature and start polymerization with … Continue. . .

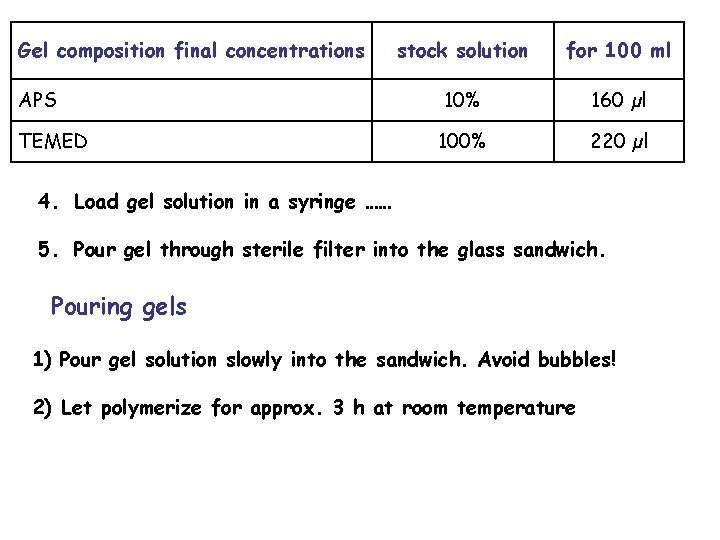
Gel composition final concentrations APS TEMED stock solution for 100 ml 10% 160 µl 100% 220 µl 4. Load gel solution in a syringe …… 5. Pour gel through sterile filter into the glass sandwich. Pouring gels 1) Pour gel solution slowly into the sandwich. Avoid bubbles! 2) Let polymerize for approx. 3 h at room temperature

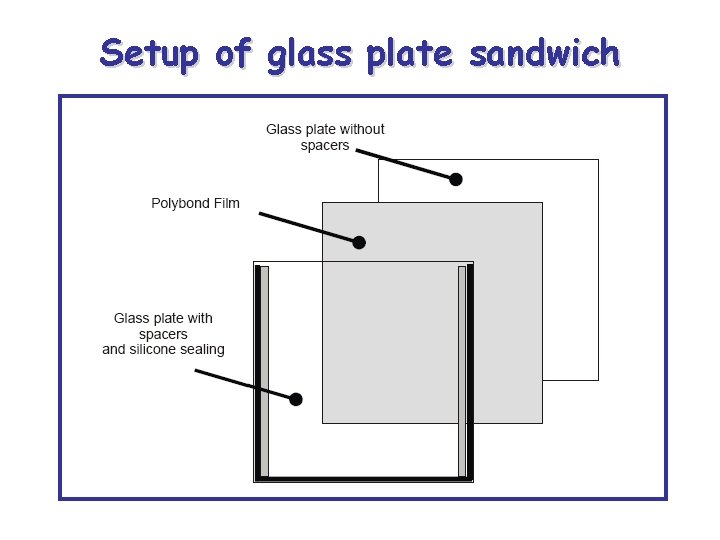
Setup of glass plate sandwich

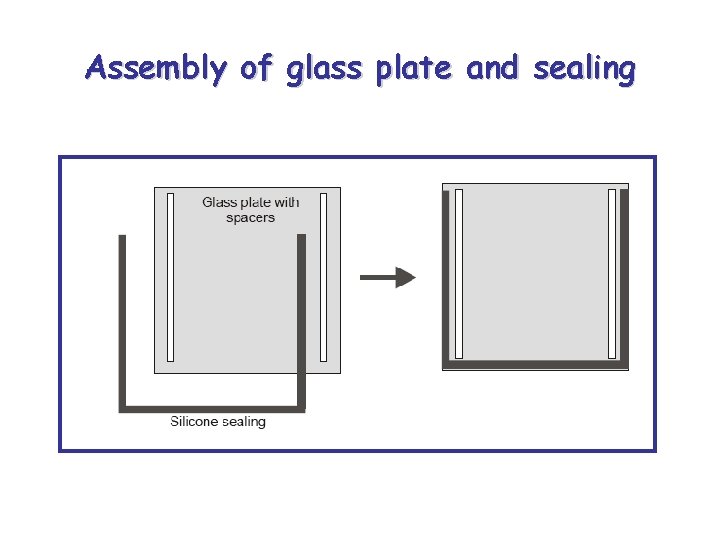
Assembly of glass plate and sealing

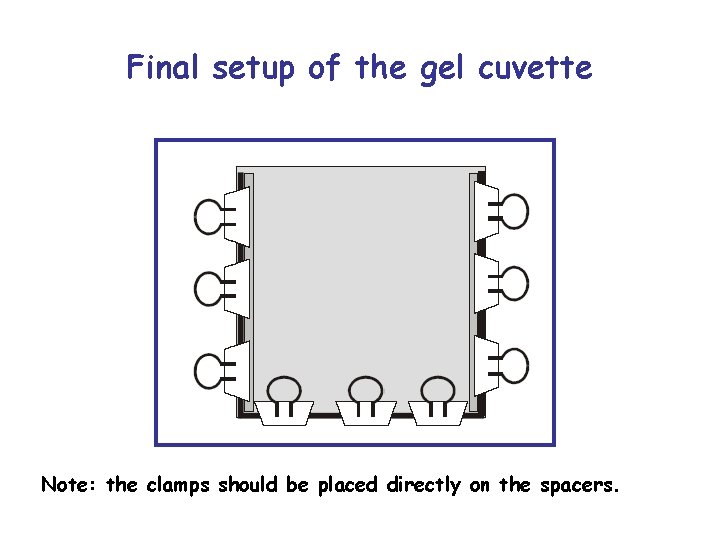
Final setup of the gel cuvette Note: the clamps should be placed directly on the spacers.

Staining q q Silver staining: the most sensitive method for detecting small amounts of DNA, RNA or proteins in polyacrylamid gels. Ethidium bromide-staining: Incubate the gel in staining solution (0. 5 g/ml ethidium bromide in 1 X TBE) for 30 - 45 min. Analyze under UV radiation