Muscular Dystrophies What is Muscular Dystrophy MD MD












- Slides: 33

Muscular Dystrophies

What is Muscular Dystrophy? (MD) • MD is an inherited muscle disease with many different forms. • In most cases muscle progressively become weaker. • Some types of MD affect voluntary muscles such as the heart. • • – – – Muscular Dystrophy: group of genetic disorders that are characterized by progressive loss of muscle integrity, wasting, and weakness. Characterized by degeneration and regeneration of muscle fibers (in contrast with static or structural myopathies) Muscular Dystrophy Association Covers all muscular dystrophies and myopathies Multisystem diseases : ALS or Friedreich Ataxia Neuropathy : HSMN, CMTD

• Muscular dystrophy refers to a group of genetic, hereditary muscle diseases that cause progressive muscle weakness. • Muscular dystrophies are characterized by progressive skeletal muscle weakness, defects in muscle proteins, and the death of muscle cells and tissue

Symptoms • • Lack of coordination Muscle weakness Progressive crippling Loss of mobility

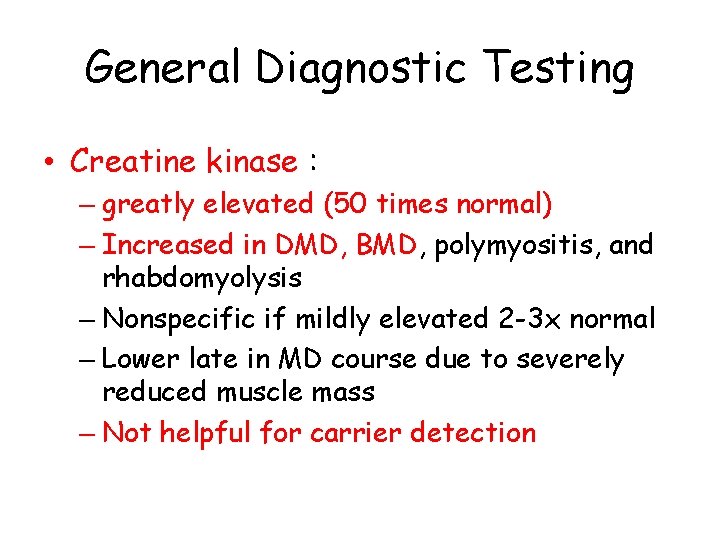
General Diagnostic Testing • Creatine kinase : – greatly elevated (50 times normal) – Increased in DMD, BMD, polymyositis, and rhabdomyolysis – Nonspecific if mildly elevated 2 -3 x normal – Lower late in MD course due to severely reduced muscle mass – Not helpful for carrier detection

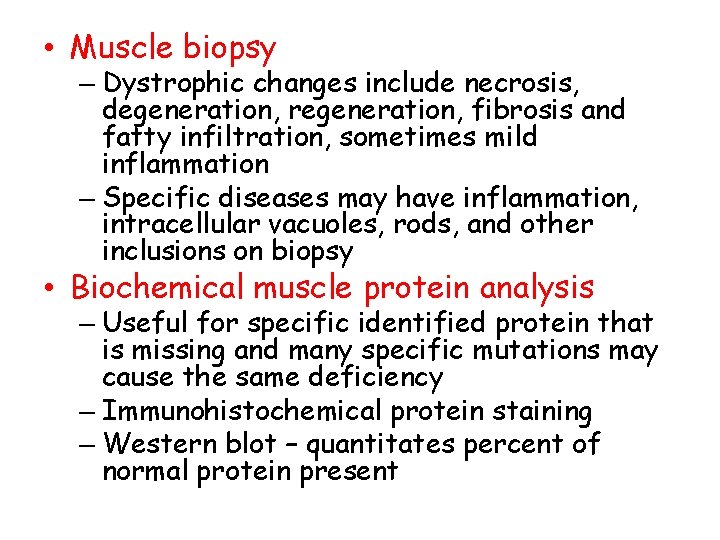
• Muscle biopsy – Dystrophic changes include necrosis, degeneration, regeneration, fibrosis and fatty infiltration, sometimes mild inflammation – Specific diseases may have inflammation, intracellular vacuoles, rods, and other inclusions on biopsy • Biochemical muscle protein analysis – Useful for specific identified protein that is missing and many specific mutations may cause the same deficiency – Immunohistochemical protein staining – Western blot – quantitates percent of normal protein present

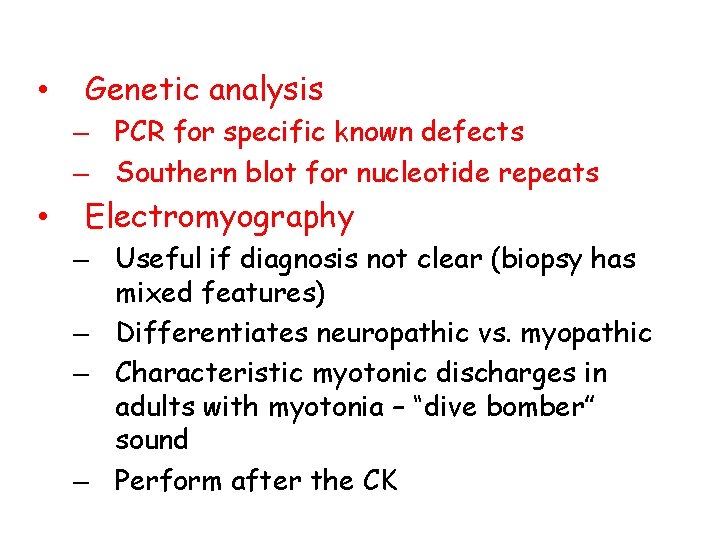
• Genetic analysis – PCR for specific known defects – Southern blot for nucleotide repeats • Electromyography – Useful if diagnosis not clear (biopsy has mixed features) – Differentiates neuropathic vs. myopathic – Characteristic myotonic discharges in adults with myotonia – “dive bomber” sound – Perform after the CK

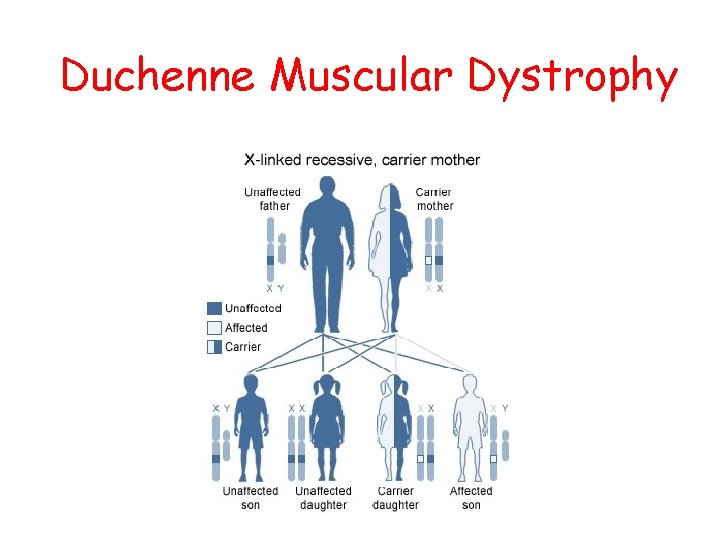
CLASSIFICATION X-linked muscular dystrophy • Duchenne muscular dystrophy • Becker muscular dystrophy • Emery-Dreifuss syndrome

CLASSIFICATION Autosomal recessive muscular dystrophy • Limb-girdle muscular dystrophy Autosomal dominant muscular dystrophy • Fascioscapulohumeral muscular dystrophy

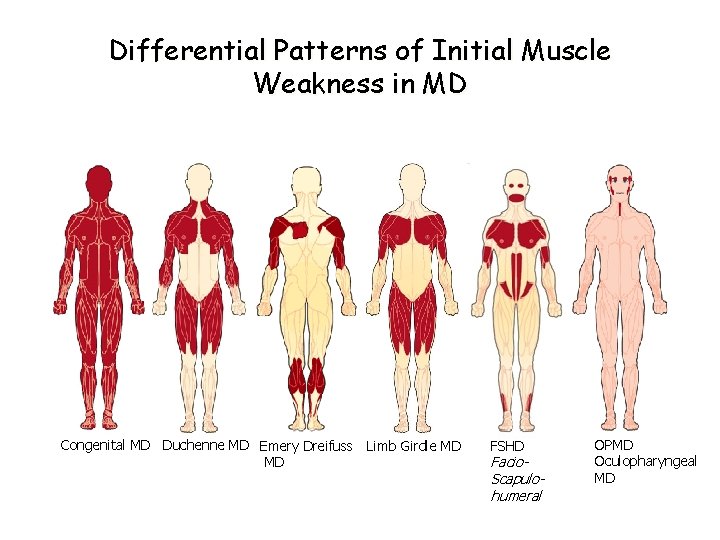
Differential Patterns of Initial Muscle Weakness in MD Congenital MD Duchenne MD Emery Dreifuss Limb Girdle MD MD FSHD Facio. Scapulohumeral OPMD Oculopharyngeal MD

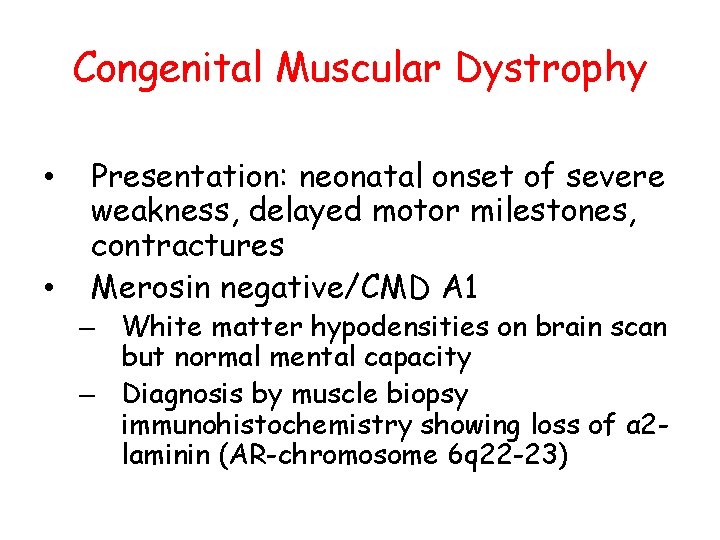
Congenital Muscular Dystrophy • • Presentation: neonatal onset of severe weakness, delayed motor milestones, contractures Merosin negative/CMD A 1 – White matter hypodensities on brain scan but normal mental capacity – Diagnosis by muscle biopsy immunohistochemistry showing loss of α 2 laminin (AR-chromosome 6 q 22 -23)

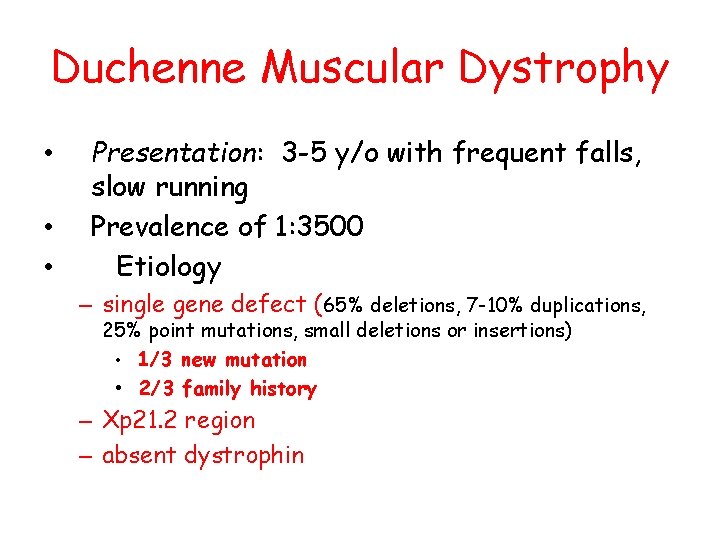
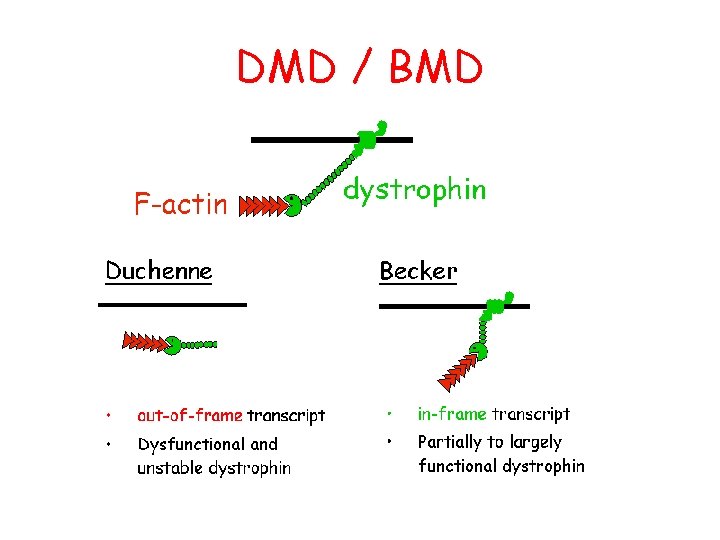
Duchenne Muscular Dystrophy • • • Presentation: 3 -5 y/o with frequent falls, slow running Prevalence of 1: 3500 Etiology – single gene defect (65% deletions, 7 -10% duplications, 25% point mutations, small deletions or insertions) • 1/3 new mutation • 2/3 family history – Xp 21. 2 region – absent dystrophin

Duchenne Muscular Dystrophy

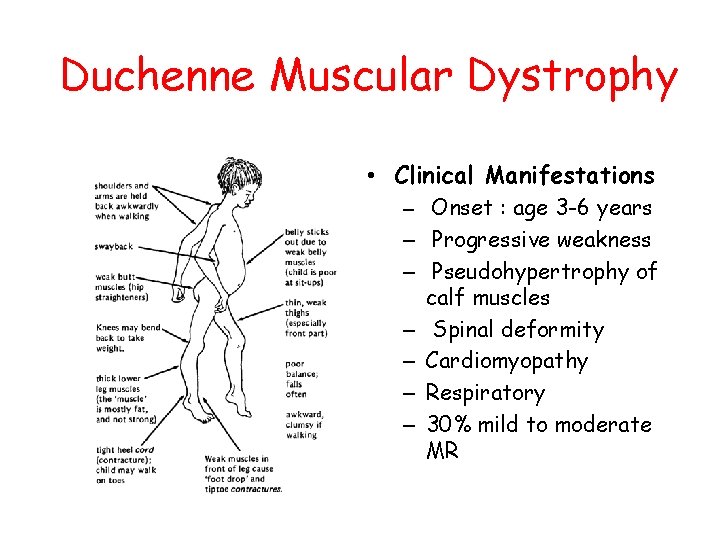
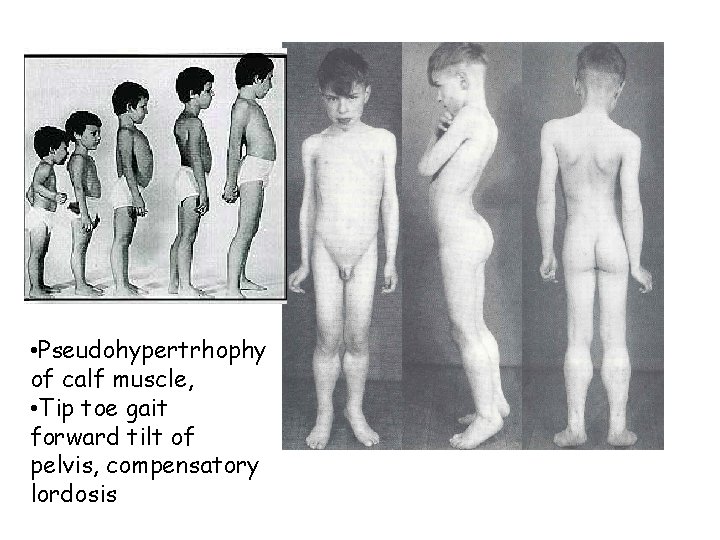
Duchenne Muscular Dystrophy • Clinical Manifestations – Onset : age 3 -6 years – Progressive weakness – Pseudohypertrophy of calf muscles – Spinal deformity – Cardiomyopathy – Respiratory – 30% mild to moderate MR

• Pseudohypertrhophy of calf muscle, • Tip toe gait forward tilt of pelvis, compensatory lordosis

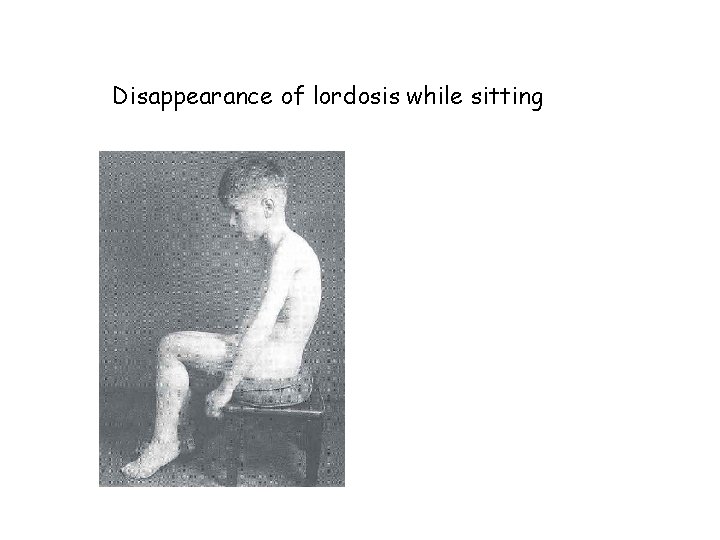
Disappearance of lordosis while sitting

GOWER’S SIGN

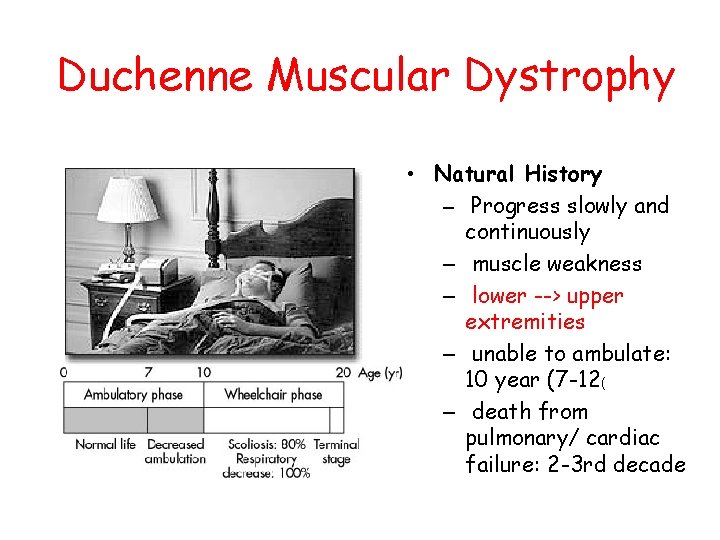
Duchenne Muscular Dystrophy • Natural History – Progress slowly and continuously – muscle weakness – lower --> upper extremities – unable to ambulate: 10 year (7 -12( – death from pulmonary/ cardiac failure: 2 -3 rd decade

Duchenne Muscular Dystrophy Diagnosis Clinical Signs (Gower’s Sign) Family history (pedigree analysis) Increase CPK (200 x( DNA mutation analysis (65%) or haplotype analysis( • Myopathic change in EMG Bx: m. degeneration • Muscle biopsy and Immunoblotting: Absence dystrophin (if geneticist can’t find the mutation !!) • • Prenatal diagnosis is available

Becker Muscular Dystrophy • • Slowly progressive form with same gene affected as Duchenne MD Etiology – single gene defect – short arm X chromosome – altered size & decreased amount of dystrophin Muscle biopsy immunostaining for dystrophin with patchy staining Disorder of function or decreased amount of dystrophin rather than absence of the protein

DMD / BMD

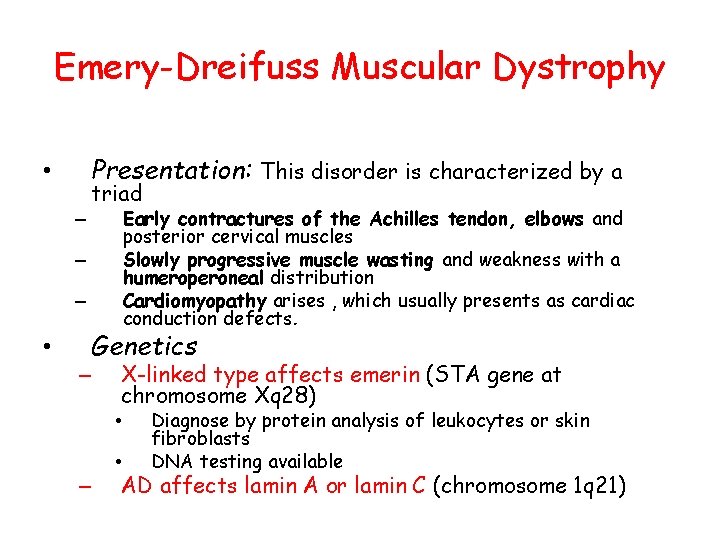
Emery-Dreifuss Muscular Dystrophy Presentation: This disorder is characterized by a • – triad Early contractures of the Achilles tendon, elbows and posterior cervical muscles Slowly progressive muscle wasting and weakness with a humeroperoneal distribution Cardiomyopathy arises , which usually presents as cardiac conduction defects. – – • Genetics – X-linked type affects emerin (STA gene at chromosome Xq 28) • • – Diagnose by protein analysis of leukocytes or skin fibroblasts DNA testing available AD affects lamin A or lamin C (chromosome 1 q 21)

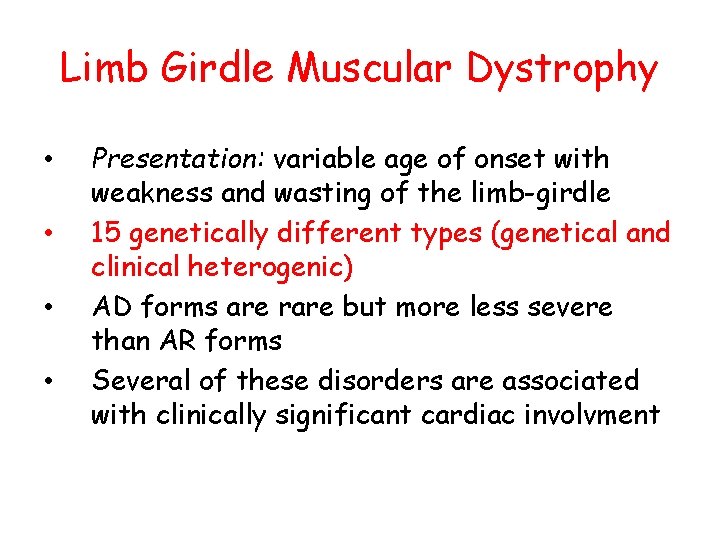
Limb Girdle Muscular Dystrophy • • Presentation: variable age of onset with weakness and wasting of the limb-girdle 15 genetically different types (genetical and clinical heterogenic) AD forms are rare but more less severe than AR forms Several of these disorders are associated with clinically significant cardiac involvment

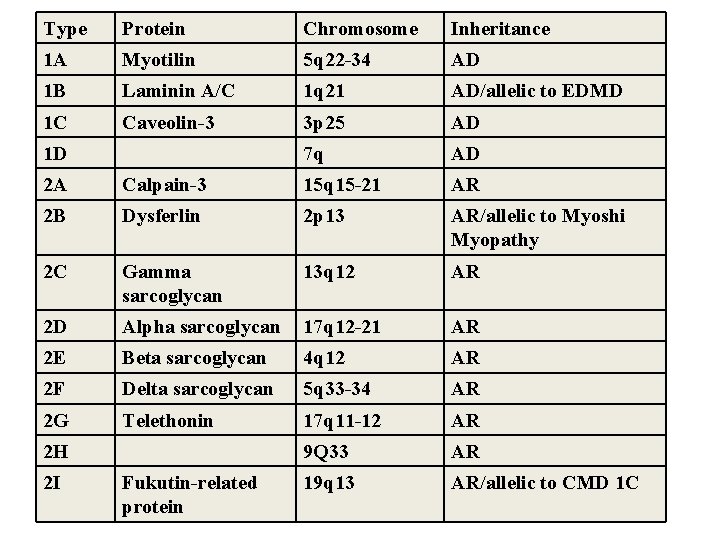
Type Protein Chromosome Inheritance 1 A Myotilin 5 q 22 -34 AD 1 B Laminin A/C 1 q 21 AD/allelic to EDMD 1 C Caveolin-3 3 p 25 AD 7 q AD 1 D 2 A Calpain-3 15 q 15 -21 AR 2 B Dysferlin 2 p 13 AR/allelic to Myoshi Myopathy 2 C Gamma sarcoglycan 13 q 12 AR 2 D Alpha sarcoglycan 17 q 12 -21 AR 2 E Beta sarcoglycan 4 q 12 AR 2 F Delta sarcoglycan 5 q 33 -34 AR 2 G Telethonin 17 q 11 -12 AR 9 Q 33 AR 19 q 13 AR/allelic to CMD 1 C 2 H 2 I Fukutin-related protein

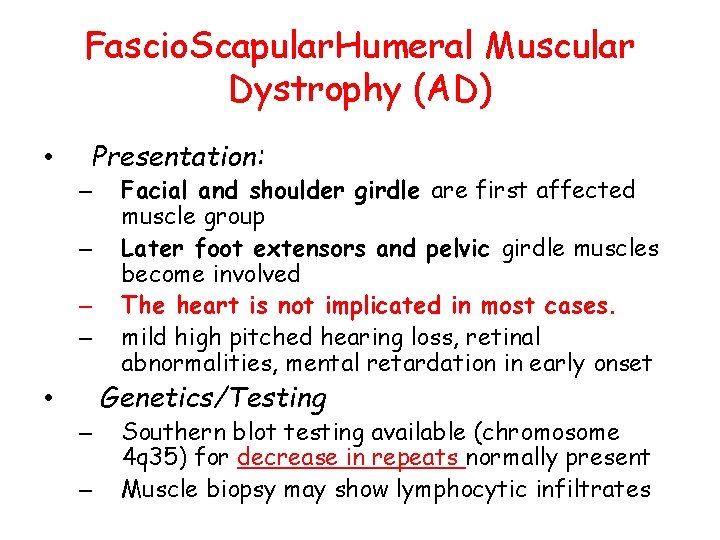
Fascio. Scapular. Humeral Muscular Dystrophy (AD) • Presentation: – – Facial and shoulder girdle are first affected muscle group Later foot extensors and pelvic girdle muscles become involved The heart is not implicated in most cases. mild high pitched hearing loss, retinal abnormalities, mental retardation in early onset Genetics/Testing • – – Southern blot testing available (chromosome 4 q 35) for decrease in repeats normally present Muscle biopsy may show lymphocytic infiltrates

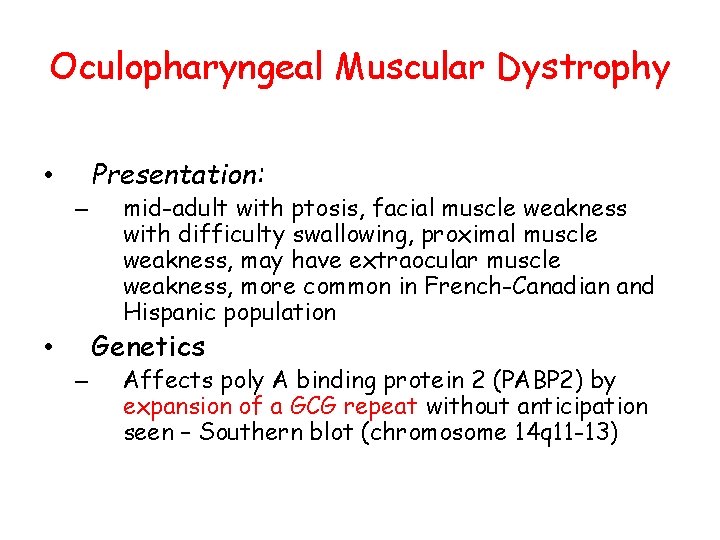
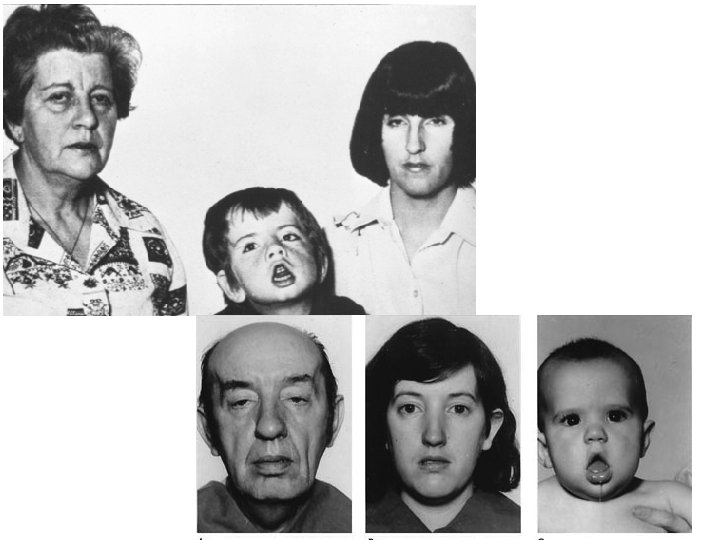
Oculopharyngeal Muscular Dystrophy Presentation: • – mid-adult with ptosis, facial muscle weakness with difficulty swallowing, proximal muscle weakness, may have extraocular muscle weakness, more common in French-Canadian and Hispanic population Genetics • – Affects poly A binding protein 2 (PABP 2) by expansion of a GCG repeat without anticipation seen – Southern blot (chromosome 14 q 11 -13)

Myotonic Dystrophy • Presentation – adult with multiple systems affected – Primarily distal and facial weakness – Facial features: frontal balding in men, ptosis, low -set ears, hatchet jaw, dysarthria, swan neck, ^ shaped upper lip – Myotonia: worse in cold weather, after age 20 – Heart: conduction block – evaluate syncope – Smooth muscle: constipation, care with swallowing, gallstones, problems with childbirth, BP lability – Brain: learning disabilities, increased sleep requirement – Ophthalmology: cataracts – Endocrine: insulin resistance, hypothyroidism, testicular atrophy


Huntington Disease • • • – – – Presentation Mood Swings Impaired cognitive functions Chorea Huntington’s Disease is an Autosomal Dominant “Trinucleotide Repeat” Disorder caused by a mutation of a gene on the 4 th chromosome which is responsible for producing the protein Huntingtin, that creates excess copies of the CAG codon which genetically program the degeneration of the neurons of the brain. Age of onset is found generally in adults around the age of 40 The earliest onset of Huntington’s ever documented was a two year old boy. The symptoms of HD can also develop at 55 or later, in which case it is harder to recognize.

The number of CAG codons varies and so does the severity of the disease – >40 repeats you develop HD, children 50% chance of developing disease – 36 -39 repeats “Grey Zone” May develop HD, children may or may not develop HD – 29 -35 repeats the individual will not develop HD, children may – <29 repeats, the individual will not develop HD, children will not develop HD

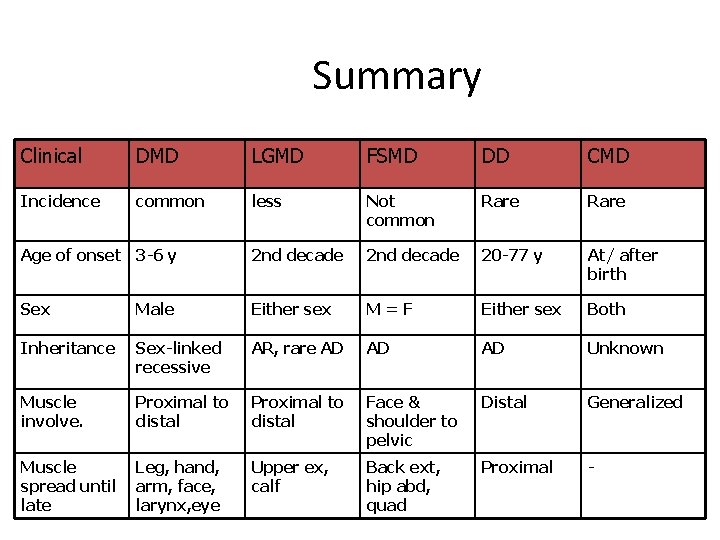
Summary Clinical DMD LGMD FSMD DD CMD Incidence common less Not common Rare Age of onset 3 -6 y 2 nd decade 20 -77 y At/ after birth Sex Male Either sex M=F Either sex Both Inheritance Sex-linked recessive AR, rare AD AD AD Unknown Muscle involve. Proximal to distal Face & shoulder to pelvic Distal Generalized Muscle spread until late Leg, hand, arm, face, larynx, eye Upper ex, calf Back ext, hip abd, quad Proximal -

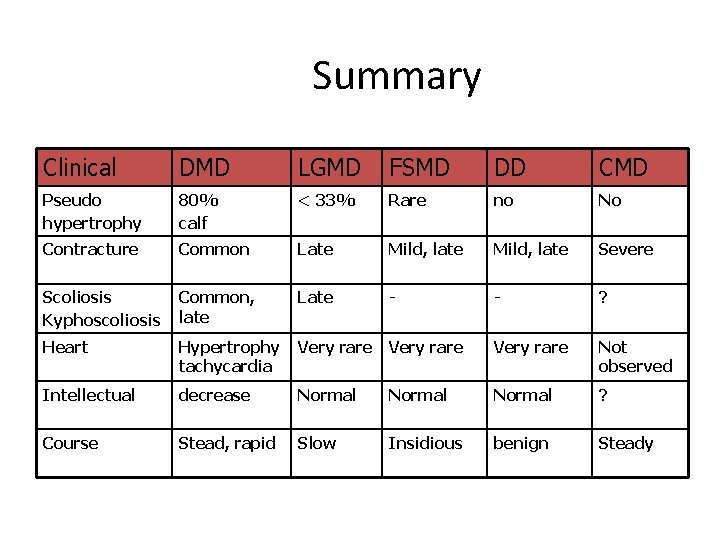
Summary Clinical DMD LGMD FSMD DD CMD Pseudo hypertrophy 80% calf < 33% Rare no No Contracture Common Late Mild, late Severe Scoliosis Kyphoscoliosis Common, late Late - - ? Heart Hypertrophy tachycardia Very rare Not observed Intellectual decrease Normal ? Course Stead, rapid Slow Insidious benign Steady

Treatment • There is no cure for MD • Medications that are prescribed for MD patients • Steroids • Braces for support • Mobility chairs • Surgery is also an option to release contractures
 Duchenne muscular dystrophy mode of inheritance
Duchenne muscular dystrophy mode of inheritance Duchenne muscular dystrophy
Duchenne muscular dystrophy Signs of duchenne muscular dystrophy
Signs of duchenne muscular dystrophy Pedigree chart dimples
Pedigree chart dimples Gowers sign
Gowers sign Emery dreifuss muscular dystrophy
Emery dreifuss muscular dystrophy Becker muscular dystrophy
Becker muscular dystrophy Duchenne muscular dystrophy
Duchenne muscular dystrophy Duchenne muscular dystrophy
Duchenne muscular dystrophy Limb girdle muscular dystrophy
Limb girdle muscular dystrophy Dr katie green
Dr katie green What is dystophin
What is dystophin Nursing diagnosis for muscular dystrophy
Nursing diagnosis for muscular dystrophy Differentiate muscular strength from muscular endurance
Differentiate muscular strength from muscular endurance Marilyn monroe always gets her man in la county
Marilyn monroe always gets her man in la county Fuchs dystrophy
Fuchs dystrophy Myotonic dystrophy type 2 vs type 1
Myotonic dystrophy type 2 vs type 1 Vulvar dystrophy thyroid
Vulvar dystrophy thyroid Kayser fleischer ring keratoconus
Kayser fleischer ring keratoconus Oil droplet reflex
Oil droplet reflex Gastronemus muscle
Gastronemus muscle 2 function of muscular system
2 function of muscular system Entrenamiento fisico
Entrenamiento fisico Chapter 7:5 muscular system
Chapter 7:5 muscular system Tono muscular
Tono muscular Anterior median plane
Anterior median plane Whats the job of the muscular system
Whats the job of the muscular system Contractile unit of muscle
Contractile unit of muscle Organo tendinoso de golgi y huso muscular
Organo tendinoso de golgi y huso muscular Muscular pectinati
Muscular pectinati Tubulos sarcoplasmicos longitudinales
Tubulos sarcoplasmicos longitudinales Estructura fina de los discos intercalares
Estructura fina de los discos intercalares Muscular tube
Muscular tube Muscular contraction
Muscular contraction