Munich Intellectual Property Law Center MIPLC Improvement Patenting









- Slides: 21

Munich Intellectual Property Law Center (MIPLC) Improvement Patenting in Pharmaceuticals – Innovative? Or Anti-innovative? Hyewon Ahn MIPLC Ph. D Candidate Sixth Advanced Research Forum on Intellectual Property Rights WIPO, 2012

Contents A. Background B. Topic of the Research C. Thesis of the Research D. Issues before Granting Patents E. Issues after Granting Patents F. Conclusion - preliminary

A. Background § Pharmaceutical Products § Originals : original drug, innovative drug, brand-name drug, reference listed drug § Generics: a drug product that is comparable to brand/reference listed drug product in dosage form, strength, route of administration, quality and performance characteristics, and intended use. 31. 05. 2012 Hyewon Ahn, MIPLC 3

A. Background § Basic / Improvement Inventions § Basic invention: a breakthrough invention providing with the roots and routes for future inventions, such as improvements, applications, and so on, and bringing out the cumulative innovations. § Improved invention: an invention as when the improvement inventor cannot innovate until the first inventor has made the basic invention. 31. 05. 2012 Hyewon Ahn, MIPLC 4

A. Background § Characteristics of Pharmaceutical Industry § Costly & lengthy R&D procedure on Innovation v. negligible ones on Imitation § Information rich molecules § Tremendous risks based on scientific, regulatory, & economic uncertainties on Innovation (“Originals”) v. much reduced uncertainty on Imitation (“Generics”) § Disconnection of choosers and payers of product Ø Patent Term Extension & Data Exclusivity 31. 05. 2012 Hyewon Ahn, MIPLC 5

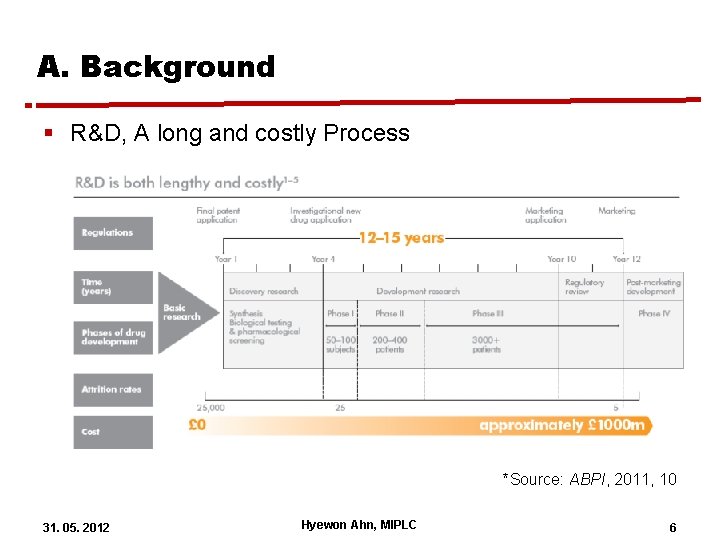
A. Background § R&D, A long and costly Process *Source: ABPI, 2011, 10 31. 05. 2012 Hyewon Ahn, MIPLC 6

A. Background § Patent Term Extension The period which can be applied and granted, to compensate for the term which needed to obtain regulatory market approvals (MAs) by authorities of new products for the pharmaceuticals & agrochemicals § Data Exclusivity The period during which the data of the original marketing authorization holder relating to (pre-) clinical tests to prove safety & efficacy of a new drug is protected, and generic applicant may not refer to the above data in their own applications of MAs. 31. 05. 2012 Hyewon Ahn, MIPLC 7

A. Background § Changes over last 10 years § Decreased R&D productivity: dearth of new medical entities (originals) § Patent cliffs on the blockbusters (e. g. : the basic patent for Lipitor expired 2011) § Frequent M&As between ‘originator’ companies and SMEs and/or ‘generic’ companies § Life cycle management, me-too or slightly me-better drugs Ø “Improvement” Inventions? 31. 05. 2012 Hyewon Ahn, MIPLC 8

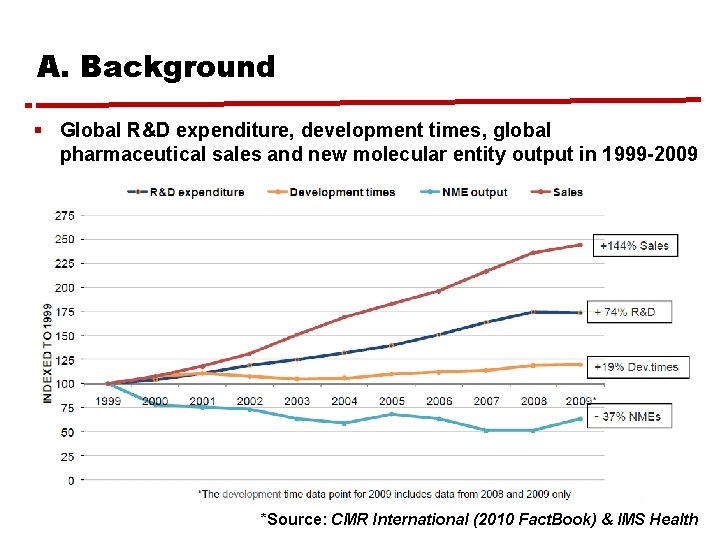
A. Background § Global R&D expenditure, development times, global pharmaceutical sales and new molecular entity output in 1999 -2009 *Source: CMR International (2010 Fact. Book) & IMS Health

B. Topic of the Research “Is allowing improvement patenting in pharmaceutical field anti-innovative? If so, what can patent law do about it? ” 31. 05. 2012 Hyewon Ahn, MIPLC 10

B. Topic of the Research § “Is allowing improvement patenting anti-innovative? If so, what can patent law do about it? ” § Definition of Improvement § Improvement: the action or process of enhancing, making or becoming greater or more complete…or better; advance or increase in value or excellence … § Selection: the action of selecting or choosing out; the fact of being selected or chosen 31. 05. 2012 Hyewon Ahn, MIPLC 11

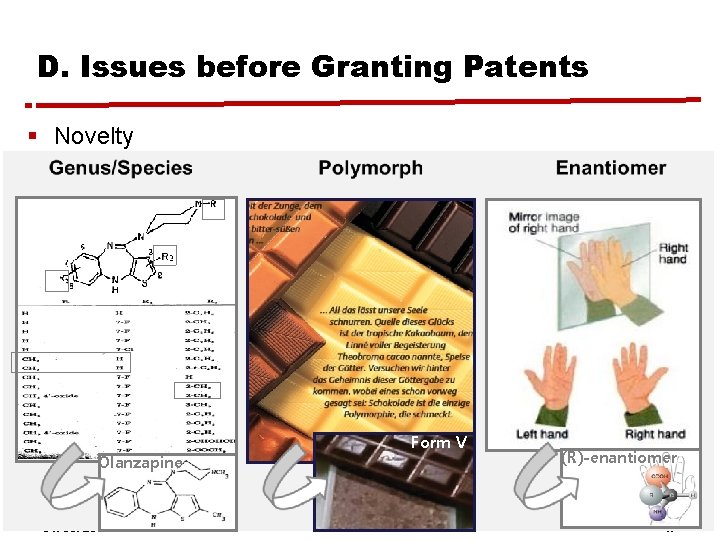
B. Topic of the Research § “Is allowing improvement patenting anti-innovative? If so, what can patent law do about it? ” § Improvement patenting in pharma: “New from Old Drug” § Improvement inventions: Esters & salts, prodrugs, formulations, combinations of active ingredients, new use/ new method of treatment § Selection inventions: genus/species, pharmacokinetic profiles, purified compound such as polymorph, metabolites, and optical isomers 31. 05. 2012 Hyewon Ahn, MIPLC 12

B. Topic of the Research § Examples of Improvement Inventions in Pharma Improvement Inventions Aspirin 31. 05. 2012 New Use Formulation Hyewon Ahn, MIPLC Combination 13

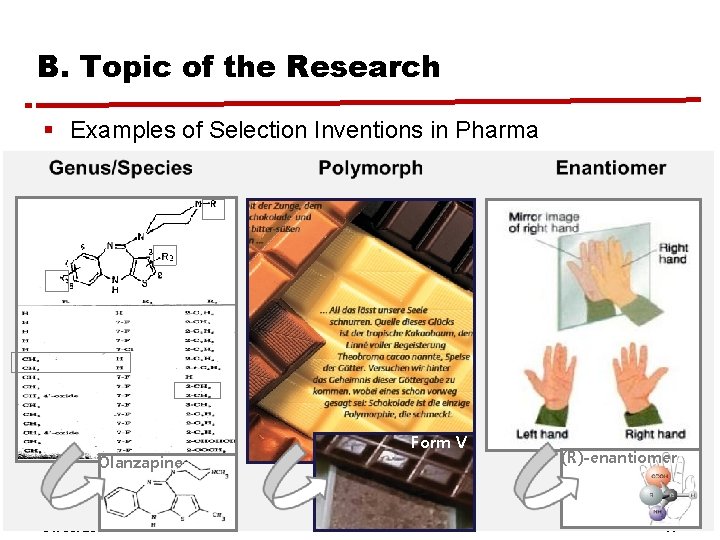
B. Topic of the Research § Examples of Selection Inventions in Pharma Olanzapine 31. 05. 2012 Form V Hyewon Ahn, MIPLC (R)-enantiomer 14

C. Thesis of the Research § Can Improvement Patenting in Pharmaceuticals § Hamper the innovation? - Lowered bar of patentability requirements for these patents - Invitation to the improvement patents? § Provide significant therapeutic advantage over Original? - Some benefits for a small subset of the patient population § Hamper the competition in this field? - Incremental advantage v new monopoly, inter alia monopoly cost - Separate patent term extensions & data exclusivities to improvement patents (esp. , by same holder as basic patentee) 31. 05. 2012 Hyewon Ahn, MIPLC 15

D. Issues before Granting Patents § Patentable Subject Matter § E. g. , Dosage Regime - Treatment of same illness of same medication in 0. 5 -1. 0 mg is patentable even if the sole distinguishing feature over the prior art (in a dose of 5 mg) is this new dosage regime (UK, DE) - “Once per day prior to sleep” of a well-know medication to treat the same illness is patentable subject matter (EPO) - Dosage regime is not patentable since it is “plainly not” a second medical indication (FR, 1 st instance) 31. 05. 2012 Hyewon Ahn, MIPLC 16

D. Issues before Granting Patents § Novelty Olanzapine 31. 05. 2012 Form V Hyewon Ahn, MIPLC (R)-enantiomer 17

D. Issues before Granting Patents § Obviousness § E. g. , Despite of the motivations, such as FDA strategies or state-of -the-art progresses to resolve the racemate, an enantiomer is not obvious because of the difficulty of separation (DE, US, UK) § Sufficiency-enablement requirement § Enablement requirement for the purpose of sufficiency § Enablement requirement for the purpose of anticipation § Implication of this discrepancy 31. 05. 2012 Hyewon Ahn, MIPLC 18

E. Issues after Granting Patents § Scope of Patents § E. g. , the scope of species selection invention is overlapping with the scope of basic invention, so far as both patents are valid. § Length of Patents § E. g. , the previously granted SPC on a racemic compound (Ofloxacin) did not hinder from granting an SPC for the enantiomer (Levofloxacin) (UK) 31. 05. 2012 Hyewon Ahn, MIPLC 19

F. Conclusion - preliminary § Yes, it is anti-innovative and counter-productive in the sense that it adds incentives to move forward to improvement inventions than to innovative inventions. § Clearer rules on patentability requirements of improvement patents, on scope of basic and improvement patent rights, on granting SPCs on improvement patents, and on utilisation of improvement patents would reduce legal uncertainties in this area. 31. 05. 2012 Hyewon Ahn, MIPLC 20

Thank you for your attention hyewon. ahn@miplc. de
 Industrial property definition
Industrial property definition Lmu munich law
Lmu munich law Ugreson maistry
Ugreson maistry Miplc
Miplc Structuralism is an intellectual movement which began in
Structuralism is an intellectual movement which began in Miplc
Miplc Miplc
Miplc Trade related aspects of intellectual property rights
Trade related aspects of intellectual property rights Intellectual property rights in professional practices
Intellectual property rights in professional practices Importance of intellectual property
Importance of intellectual property Intellectual property management definition
Intellectual property management definition Advantages of intellectual property
Advantages of intellectual property Example of intellectual property in business plan
Example of intellectual property in business plan Intellectual property in computer ethics
Intellectual property in computer ethics Right to intellectual property of teachers
Right to intellectual property of teachers Incentive theory
Incentive theory Concept of intellectual property
Concept of intellectual property Valuing intangible assets
Valuing intangible assets Intellectual property rights
Intellectual property rights Intellectual property rights
Intellectual property rights Characteristics of intellectual property
Characteristics of intellectual property Discuss intellectual property frankly
Discuss intellectual property frankly




































