Multivalent aka Transition Metals Formula to Name Definition





- Slides: 23

Multivalent (aka Transition) Metals Formula to Name

Definition Multivalent Metal- metal that has more than one possibility for cationic charge The Appendix of your book (Page A-2) has the following chart Common multivalent metals and their charges Cobalt Copper Iron Lead Manganese Mercury Tin Co+2 Cu+1 Fe+2 Pb+2 Mn+2 Hg 2+2 Sn+2 Co+3 Cu+2 Fe+3 Pb+4 Mn+3 Hg+2 Sn+4

Identifying & Naming Multivalent (Transition) Metals • These compounds have: – One of the multivalent (transition) metals in that chart • To name these compounds: – Write the name of the metal element (cation) – Write the name of the anion (element name with “-ide” or polyatomic ion name) – Determine the total negative charge – Total negative charge = total positive charge for all neutral compounds – Determine the charge on each metal atom – Write the charge in roman numerals in parenthesis after the metal’s name

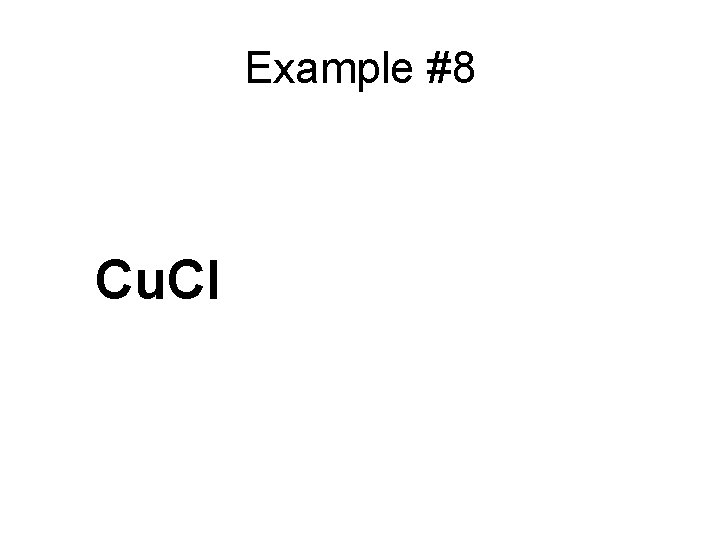
Example #8 Cu. Cl

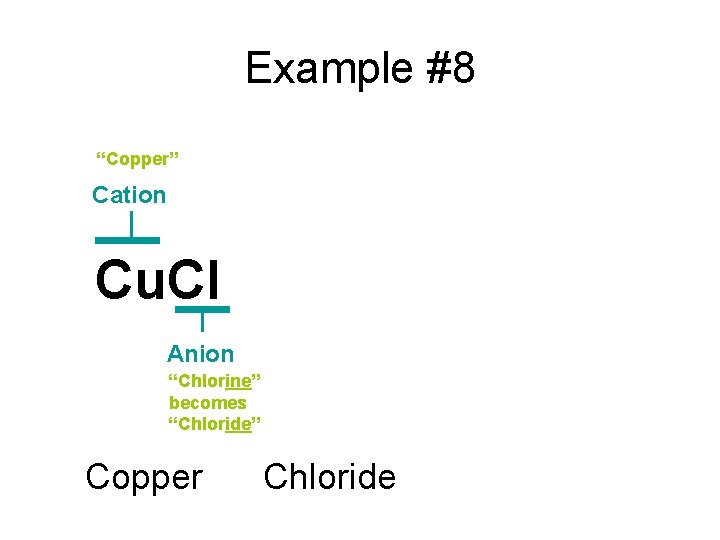
Example #8 “Copper” Cation Cu. Cl Anion “Chlorine” becomes “Chloride” Copper Chloride

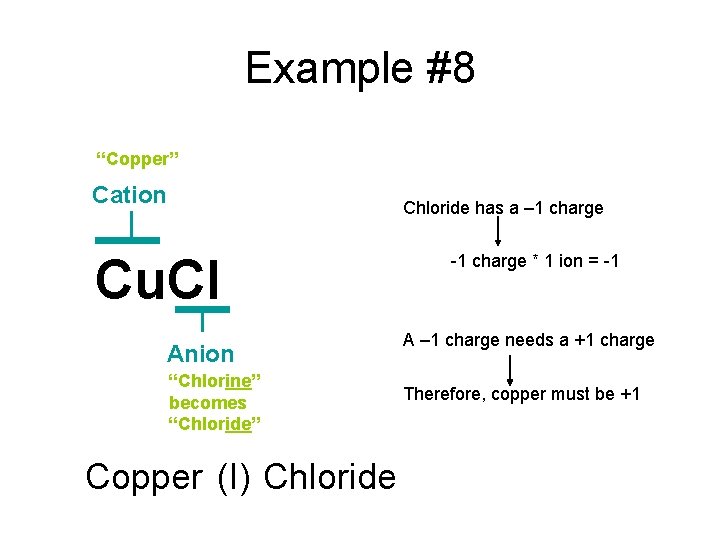
Example #8 “Copper” Cation Chloride has a – 1 charge Cu. Cl Anion “Chlorine” becomes “Chloride” Copper (I) Chloride -1 charge * 1 ion = -1 A – 1 charge needs a +1 charge Therefore, copper must be +1

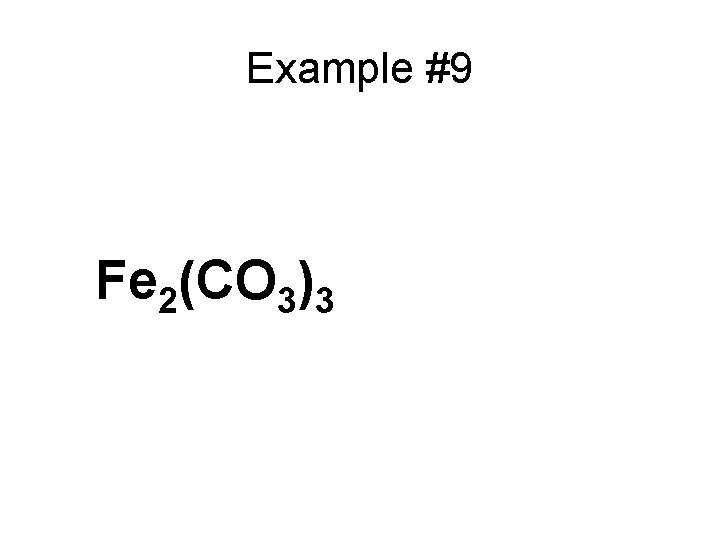
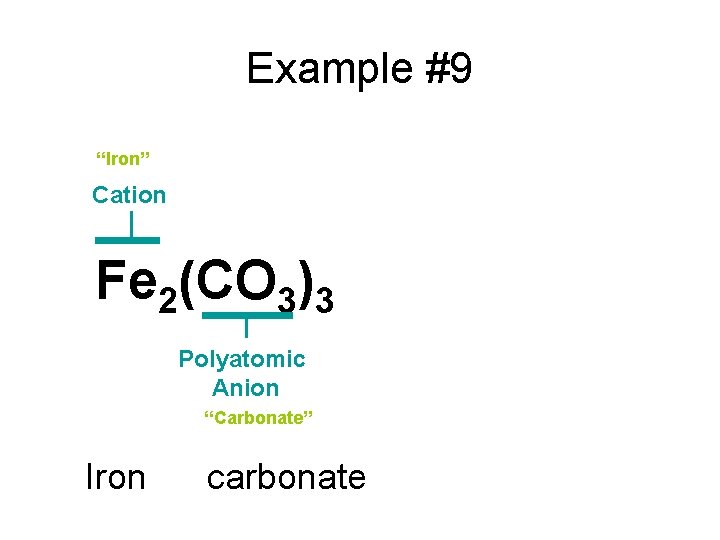
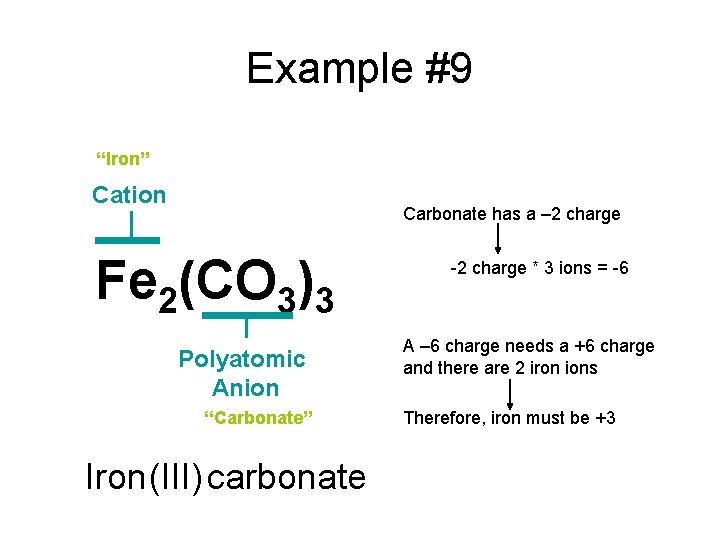
Example #9 Fe 2(CO 3)3

Example #9 “Iron” Cation Fe 2(CO 3)3 Polyatomic Anion “Carbonate” Iron carbonate

Example #9 “Iron” Cation Carbonate has a – 2 charge Fe 2(CO 3)3 Polyatomic Anion “Carbonate” Iron (III) carbonate -2 charge * 3 ions = -6 A – 6 charge needs a +6 charge and there are 2 iron ions Therefore, iron must be +3

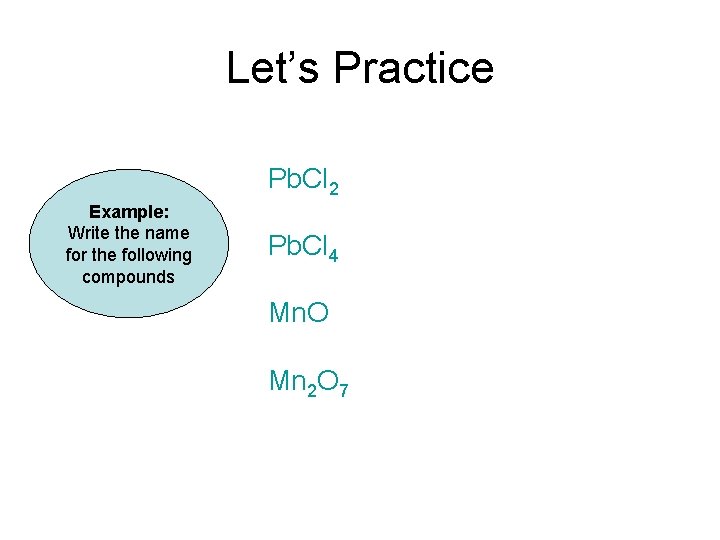
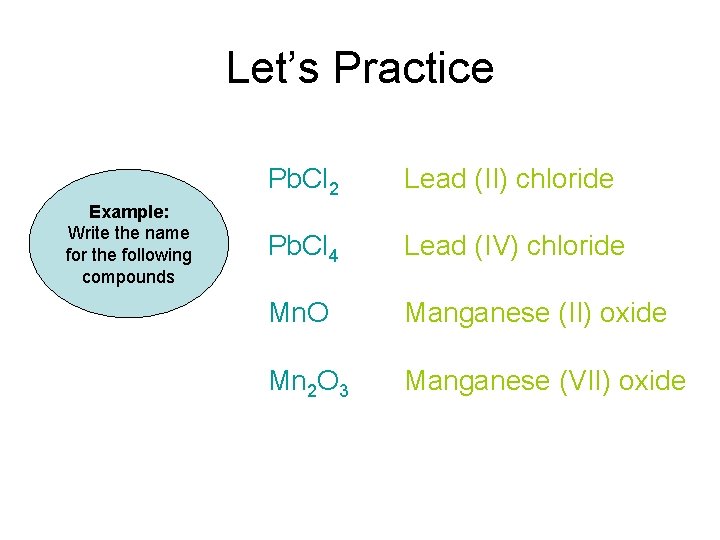
Let’s Practice Pb. Cl 2 Example: Write the name for the following compounds Pb. Cl 4 Mn. O Mn 2 O 7

Let’s Practice Example: Write the name for the following compounds Pb. Cl 2 Lead (II) chloride Pb. Cl 4 Lead (IV) chloride Mn. O Manganese (II) oxide Mn 2 O 3 Manganese (VII) oxide

Reminders from Section 2. 2 • Your Appendix (Page A-2) has lists of: – Common polyatomic ions – Multivalent metals – Covalent prefixes • Use your periodic table to determine the charges of common elements when they form ions

Multivalent Metals Name to Formula

Definition Multivalent Metal- metal that has more than one possibility for cationic charge

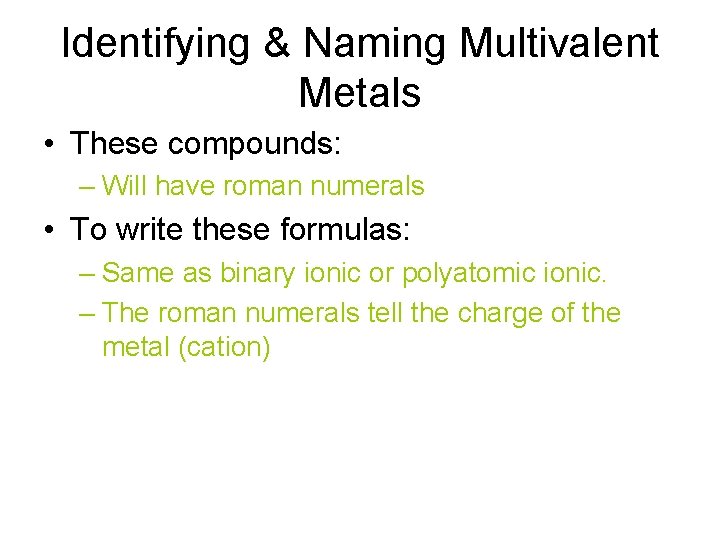
Identifying & Naming Multivalent Metals • These compounds: – Will have roman numerals • To write these formulas: – Same as binary ionic or polyatomic ionic. – The roman numerals tell the charge of the metal (cation)

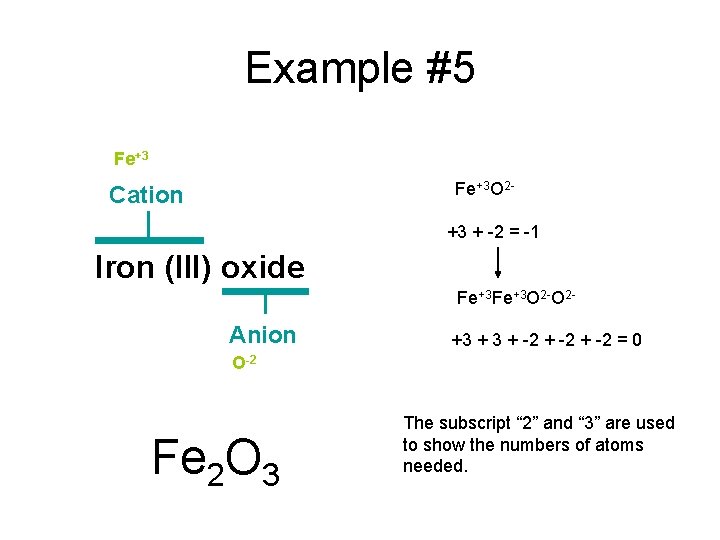
Example #5 Iron (III) oxide

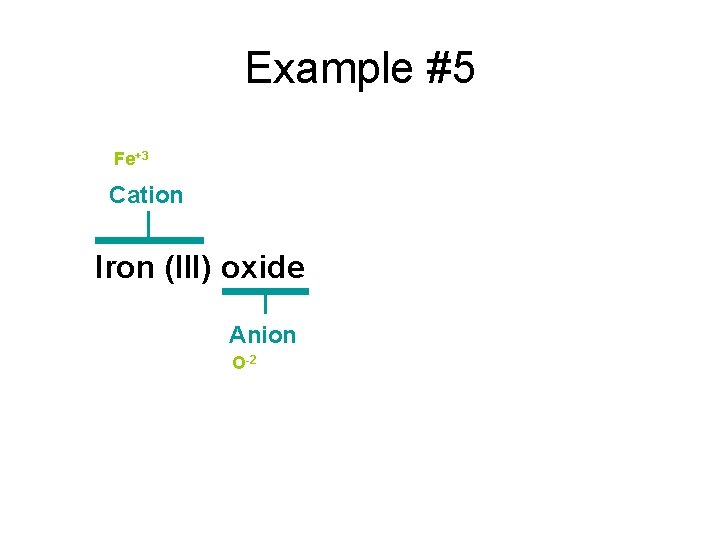
Example #5 Fe+3 Cation Iron (III) oxide Anion O-2

Example #5 Fe+3 O 2 - Cation +3 + -2 = -1 Iron (III) oxide Fe+3 O 2 - Anion +3 + -2 = 0 O-2 Fe 2 O 3 The subscript “ 2” and “ 3” are used to show the numbers of atoms needed.

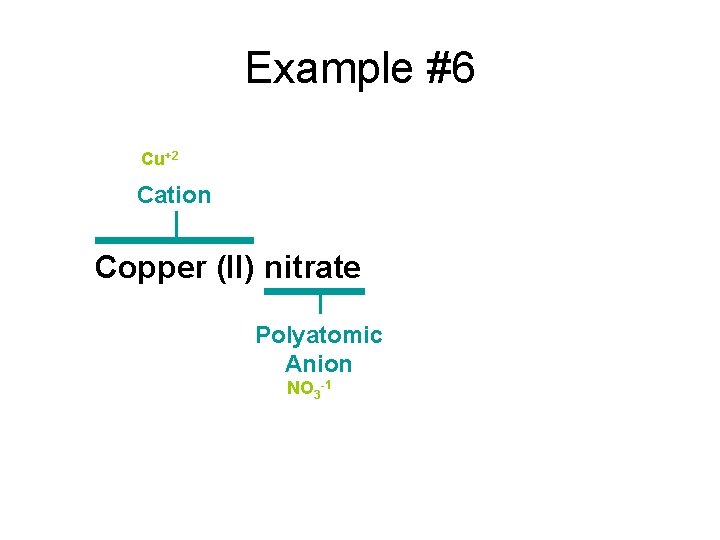
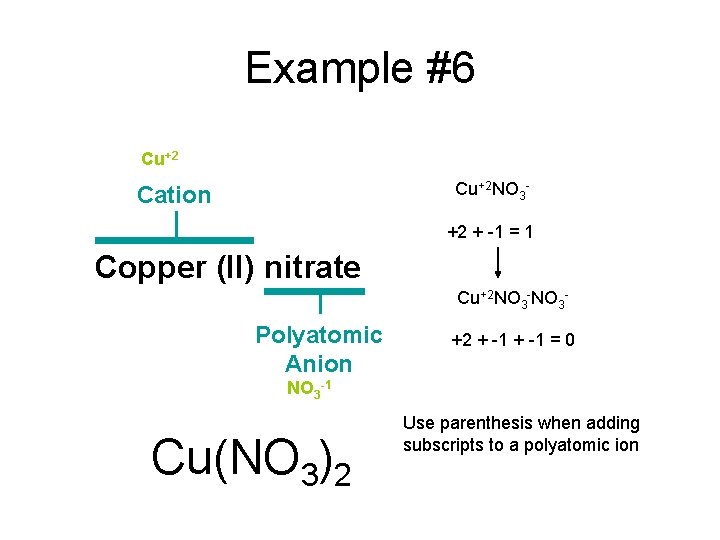
Example #6 Copper (II) nitrate

Example #6 Cu+2 Cation Copper (II) nitrate Polyatomic Anion NO 3 -1

Example #6 Cu+2 NO 3 - Cation +2 + -1 = 1 Copper (II) nitrate Cu+2 NO 3 - Polyatomic Anion +2 + -1 = 0 NO 3 -1 Cu(NO 3)2 Use parenthesis when adding subscripts to a polyatomic ion

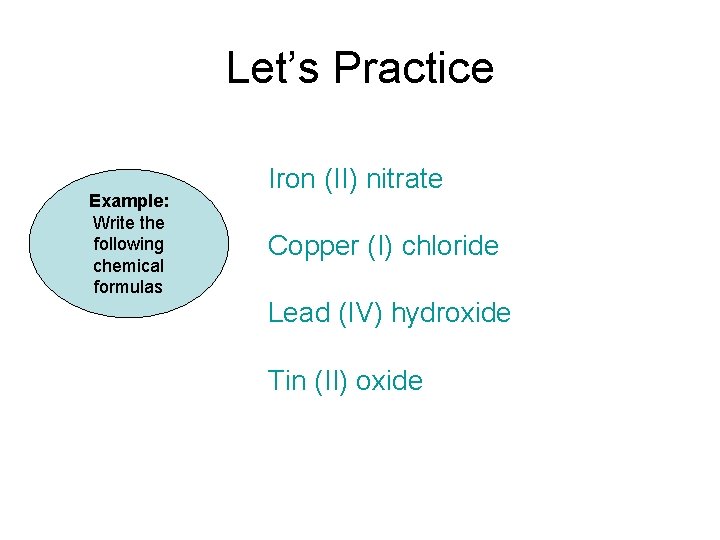
Let’s Practice Example: Write the following chemical formulas Iron (II) nitrate Copper (I) chloride Lead (IV) hydroxide Tin (II) oxide

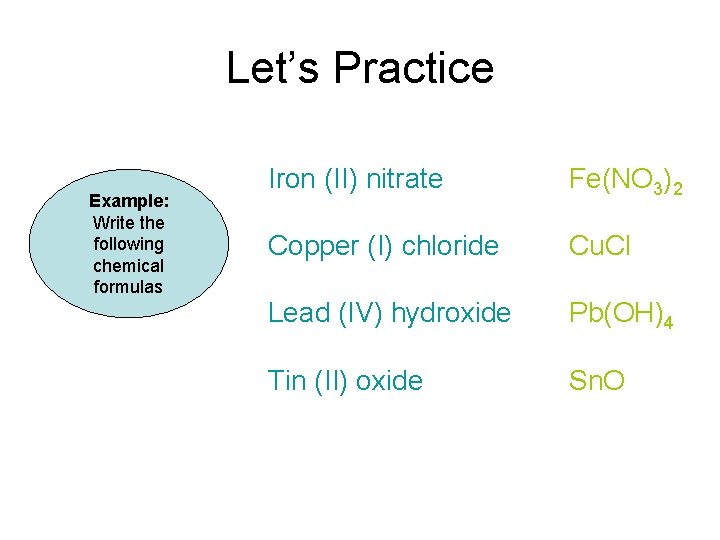
Let’s Practice Example: Write the following chemical formulas Iron (II) nitrate Fe(NO 3)2 Copper (I) chloride Cu. Cl Lead (IV) hydroxide Pb(OH)4 Tin (II) oxide Sn. O