Multitest strategies for the determination of HIV serostatus




- Slides: 19

Multi-test strategies for the determination of HIV serostatus without the use of Western immunoblotting New strategies for HIV diagnostic algorithms Jane Feldman, Susan Wells, Michele Owen, and Tim Granade

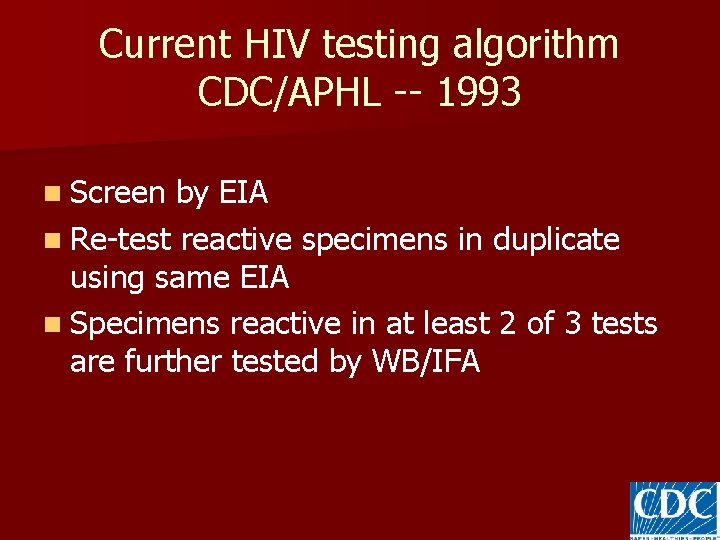
Current HIV testing algorithm CDC/APHL -- 1993 n Screen by EIA n Re-test reactive specimens in duplicate using same EIA n Specimens reactive in at least 2 of 3 tests are further tested by WB/IFA

Changes in HIV diagnostic testing n Introduction of 3 rd and 4 th generation HIV EIAs n FDA approval of rapid HIV antibody screening tests n Use of HIV nucleic acid detection assays Can alternative strategies provide equivalent or better results?

Methods n Samples (n=342) that were submitted to the CDC HIV CLIA Reference Laboratory – “Difficult” samples § Most submitted due to inability of public health labs to reach a definitive answer § Suspicion of HIV-2 – Many likely screened with Bio-Rad HIV 1/2 plus O

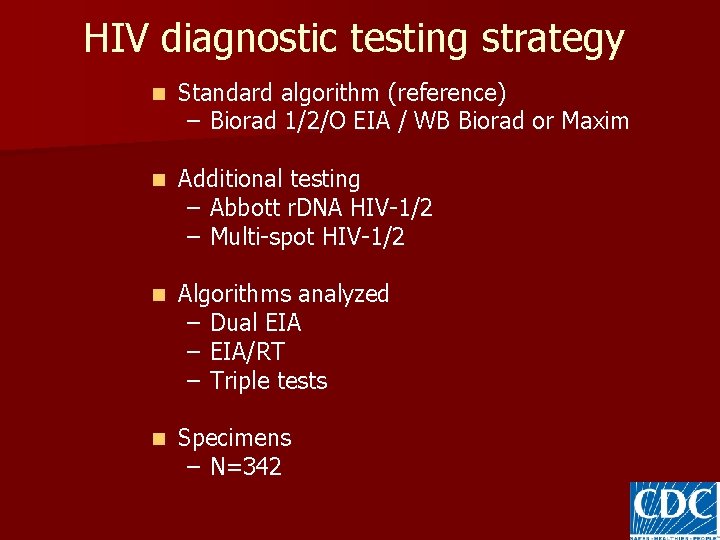
HIV diagnostic testing strategy n Standard algorithm (reference) – Biorad 1/2/O EIA / WB Biorad or Maxim n Additional testing – Abbott r. DNA HIV-1/2 – Multi-spot HIV-1/2 n Algorithms analyzed – Dual EIA – EIA/RT – Triple tests n Specimens – N=342

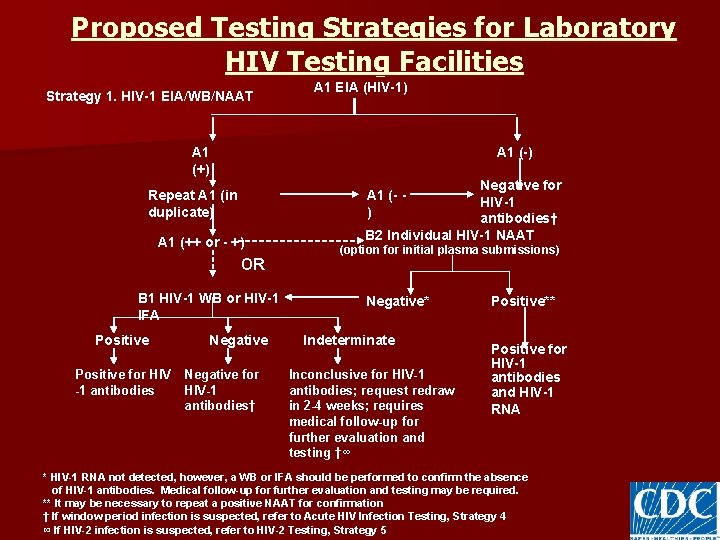
Proposed Testing Strategies for Laboratory HIV Testing Facilities Strategy 1. HIV-1 EIA/WB/NAAT A 1 EIA (HIV-1) A 1 (+) A 1 (-) Repeat A 1 (in duplicate) A 1 (++ or - +) OR B 1 HIV-1 WB or HIV-1 IFA Positive for HIV -1 antibodies Negative for HIV-1 antibodies† B 2 Individual HIV-1 NAAT A 1 (- ) Negative for HIV-1 antibodies† (option for initial plasma submissions) Negative* Indeterminate Inconclusive for HIV-1 antibodies; request redraw in 2 -4 weeks; requires medical follow-up for further evaluation and testing †∞ Positive** Positive for HIV-1 antibodies and HIV-1 RNA * HIV-1 RNA not detected, however, a WB or IFA should be performed to confirm the absence of HIV-1 antibodies. Medical follow-up for further evaluation and testing may be required. ** It may be necessary to repeat a positive NAAT for confirmation † If window period infection is suspected, refer to Acute HIV Infection Testing, Strategy 4 ∞ If HIV-2 infection is suspected, refer to HIV-2 Testing, Strategy 5

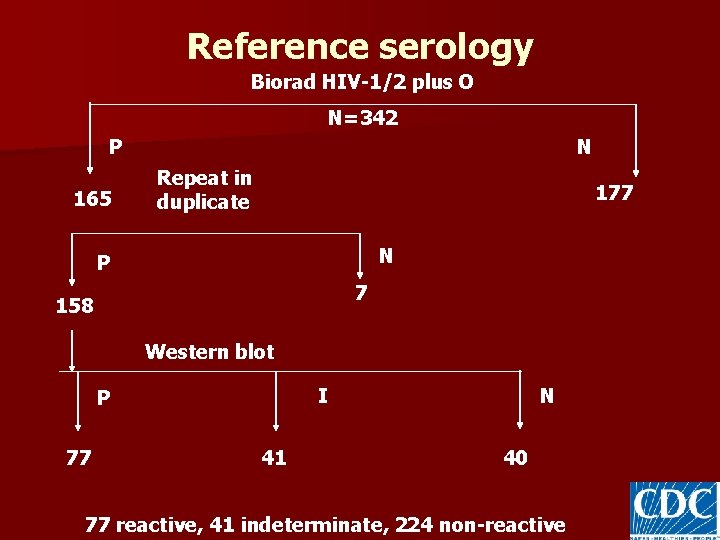
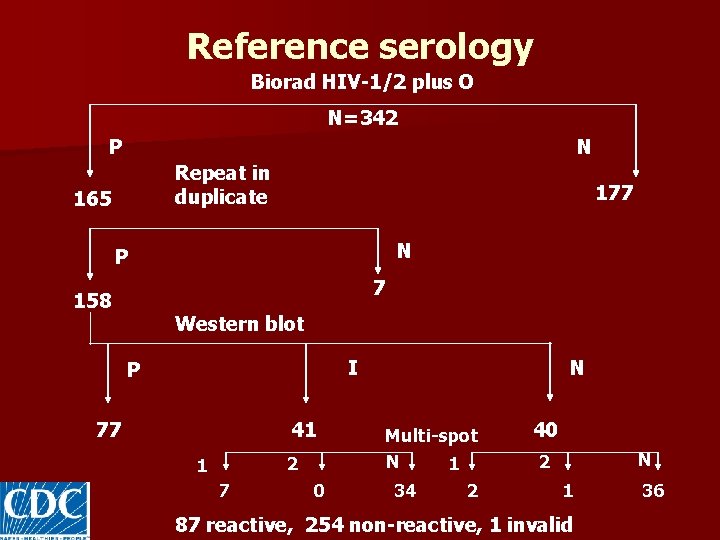
Reference serology Biorad HIV-1/2 plus O N=342 P 165 N Repeat in duplicate 177 N P 7 158 Western blot I P 77 41 N 40 77 reactive, 41 indeterminate, 224 non-reactive

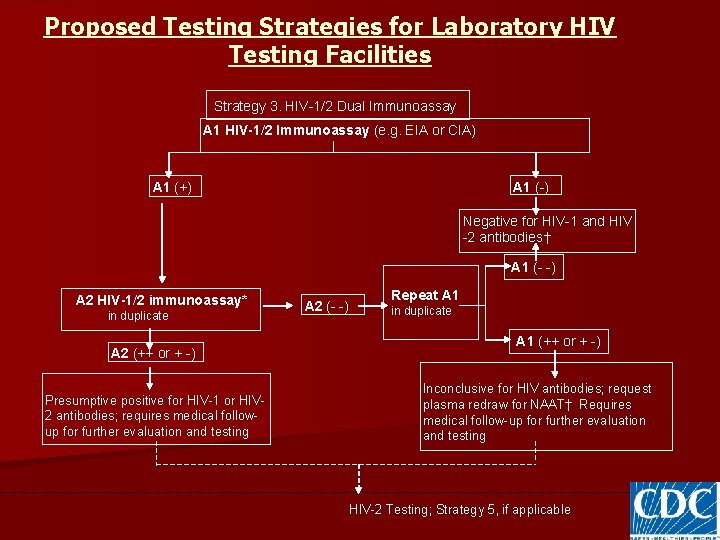
Proposed Testing Strategies for Laboratory HIV Testing Facilities Strategy 3. HIV-1/2 Dual Immunoassay A 1 HIV-1/2 Immunoassay (e. g. EIA or CIA) A 1 (+) A 1 (-) Negative for HIV-1 and HIV -2 antibodies† A 1 (- -) A 2 HIV-1/2 immunoassay* in duplicate A 2 (++ or + -) Presumptive positive for HIV-1 or HIV 2 antibodies; requires medical followup for further evaluation and testing A 2 (- -) Repeat A 1 in duplicate A 1 (++ or + -) Inconclusive for HIV antibodies; request plasma redraw for NAAT† Requires medical follow-up for further evaluation and testing HIV-2 Testing; Strategy 5, if applicable

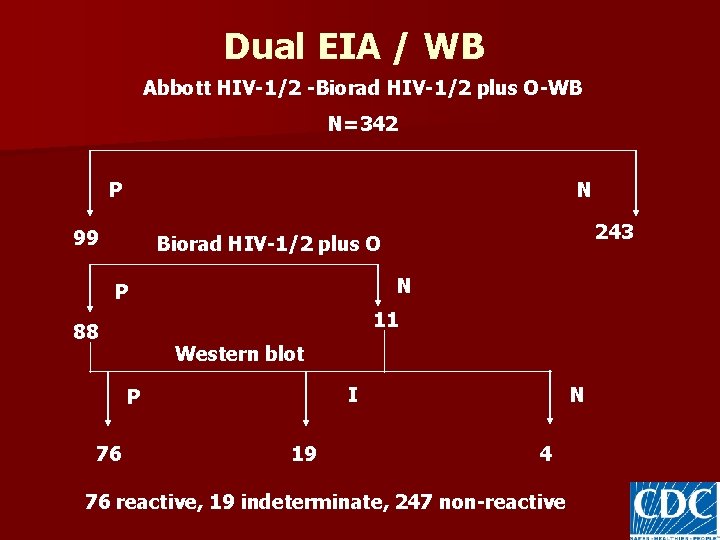
Dual EIA / WB Abbott HIV-1/2 -Biorad HIV-1/2 plus O-WB N=342 P N 99 243 Biorad HIV-1/2 plus O N P 11 88 Western blot I P 76 19 N 4 76 reactive, 19 indeterminate, 247 non-reactive

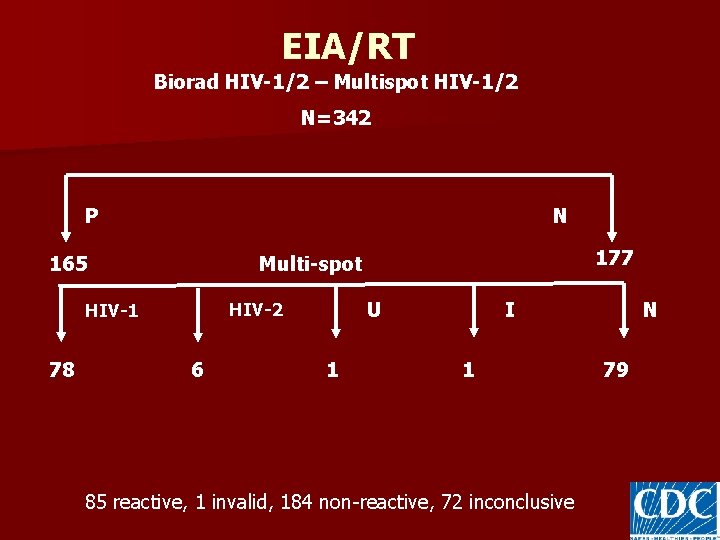
EIA/RT Biorad HIV-1/2 – Multispot HIV-1/2 N=342 P N 165 U HIV-2 HIV-1 78 177 Multi-spot 6 1 N I 1 85 reactive, 1 invalid, 184 non-reactive, 72 inconclusive 79

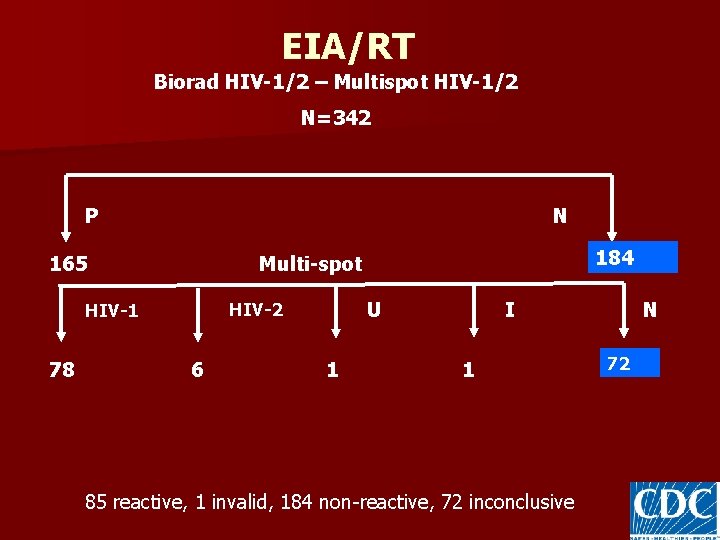
EIA/RT Biorad HIV-1/2 – Multispot HIV-1/2 N=342 P N 165 U HIV-2 HIV-1 78 184 Multi-spot 6 1 N I 1 85 reactive, 1 invalid, 184 non-reactive, 72 inconclusive 72

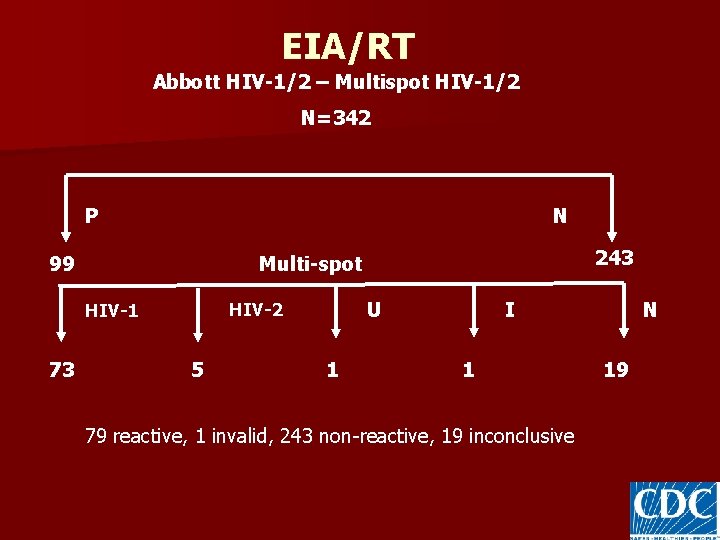
EIA/RT Abbott HIV-1/2 – Multispot HIV-1/2 N=342 P N 99 U HIV-2 HIV-1 73 243 Multi-spot 5 1 N I 1 79 reactive, 1 invalid, 243 non-reactive, 19 inconclusive 19

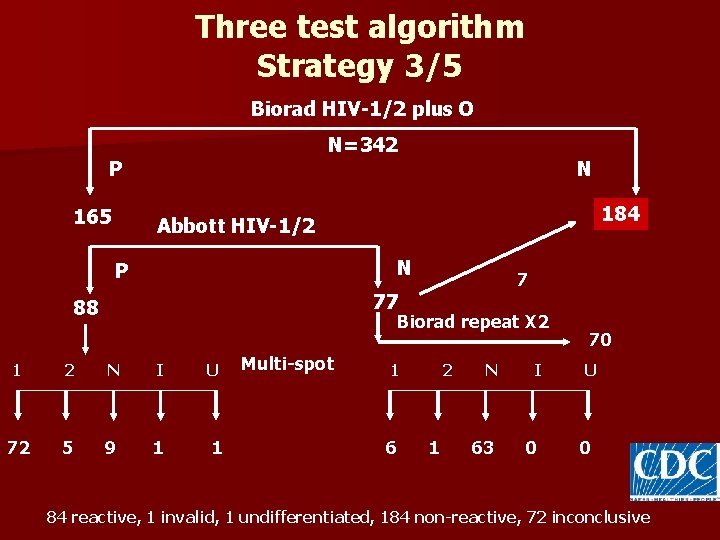
Three test algorithm Strategy 3/5 Biorad HIV-1/2 plus O N=342 P 165 N 177 184 Abbott HIV-1/2 N P 7 77 88 Biorad repeat X 2 1 2 N I U 72 5 9 1 1 Multi-spot 1 6 2 1 N 63 I 0 70 U 0 84 reactive, 1 invalid, 1 undifferentiated, 184 non-reactive, 72 inconclusive

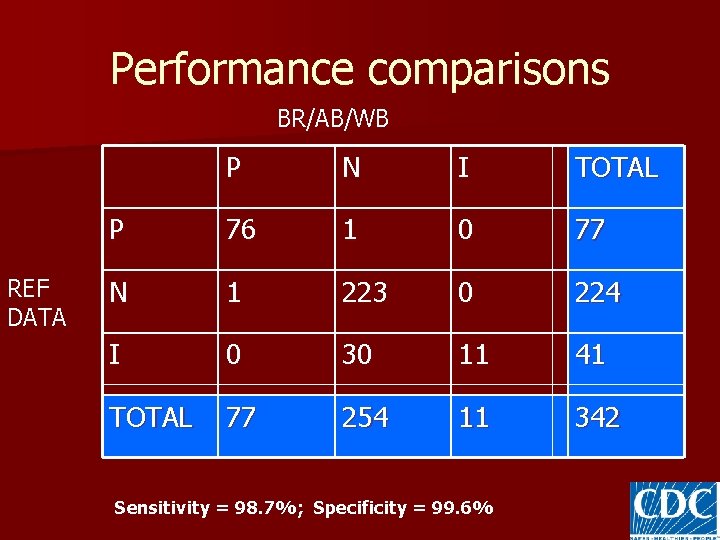
Performance comparisons BR/AB/WB REF DATA P N I TOTAL P 76 1 0 77 N 1 223 0 224 I 0 30 11 41 TOTAL 77 254 11 342 Sensitivity = 98. 7%; Specificity = 99. 6%

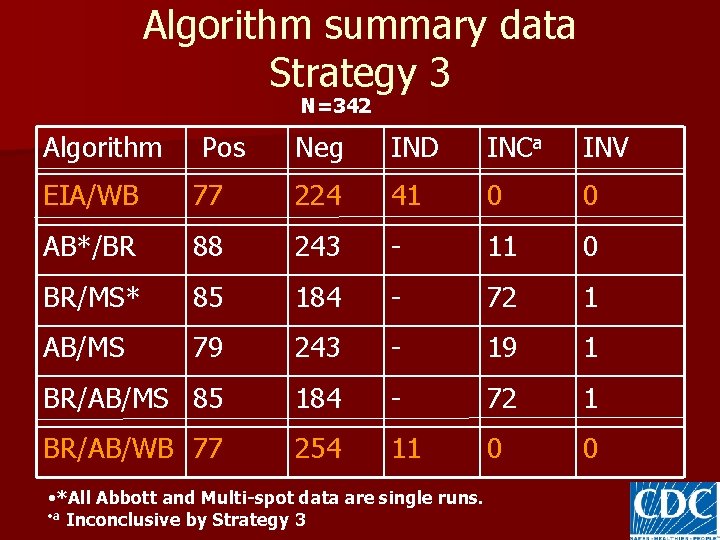
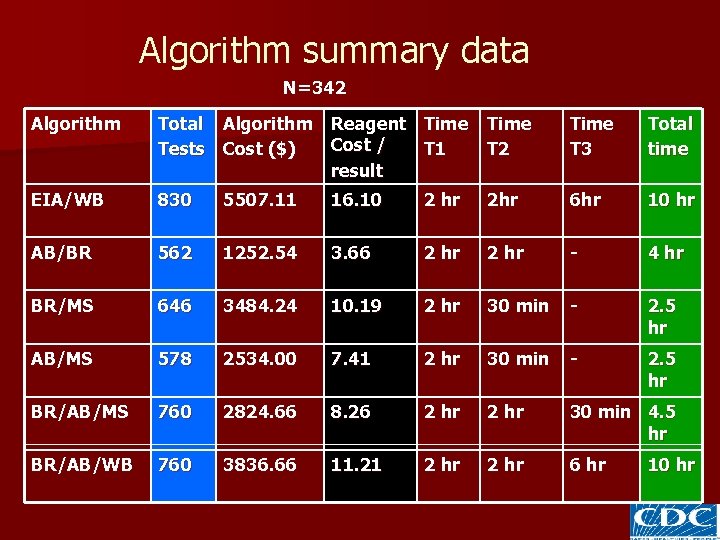
Algorithm summary data Strategy 3 N=342 Algorithm Pos Neg IND INCa INV EIA/WB 77 224 41 0 0 AB*/BR 88 243 - 11 0 BR/MS* 85 184 - 72 1 AB/MS 79 243 - 19 1 BR/AB/MS 85 184 - 72 1 BR/AB/WB 77 254 11 0 0 • *All Abbott and Multi-spot data are single runs. • a Inconclusive by Strategy 3

Algorithm summary data N=342 Algorithm Total Algorithm Reagent Time Cost / Tests Cost ($) T 1 result Time T 2 Time T 3 Total time EIA/WB 830 5507. 11 16. 10 2 hr 2 hr 6 hr 10 hr AB/BR 562 1252. 54 3. 66 2 hr - 4 hr BR/MS 646 3484. 24 10. 19 2 hr 30 min - 2. 5 hr AB/MS 578 2534. 00 7. 41 2 hr 30 min - 2. 5 hr BR/AB/MS 760 2824. 66 8. 26 2 hr 30 min 4. 5 hr BR/AB/WB 760 3836. 66 11. 21 2 hr 6 hr 10 hr

Reference serology Biorad HIV-1/2 plus O N=342 P N Repeat in duplicate 165 177 N P 7 158 Western blot I P 77 41 2 1 7 0 N Multi-spot N 1 34 2 40 N 2 1 87 reactive, 254 non-reactive, 1 invalid 36

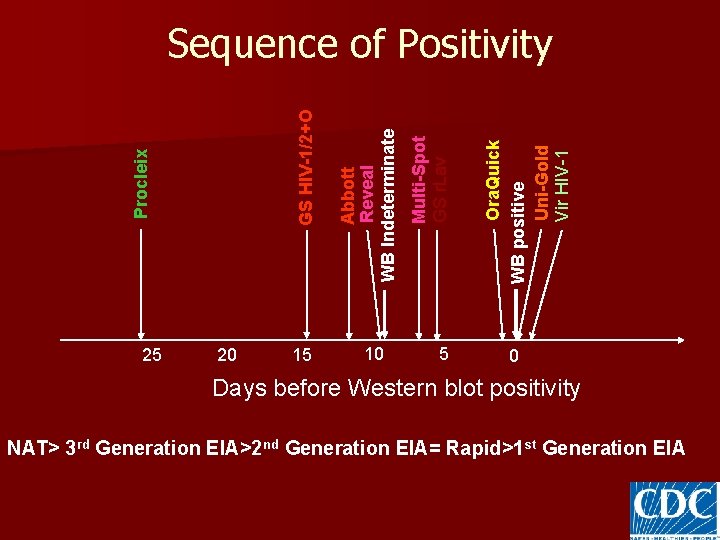
25 20 15 10 5 Ora. Quick WB positive Uni-Gold Vir HIV-1 Multi-Spot GS r. Lav Abbott Reveal WB Indeterminate Procleix GS HIV-1/2+O Sequence of Positivity 0 Days before Western blot positivity NAT> 3 rd Generation EIA>2 nd Generation EIA= Rapid>1 st Generation EIA

Acknowledgements Jane Feldman Susan Wells Michele Owen Patrick Sprinkle