MULTISTIX 10 SG with CLINITEK Status Connect InService





























- Slides: 46

MULTISTIX 10 SG with CLINITEK Status Connect In-Service Training Maria Peluso-Lapsley, CDM Marketing October 2018, HOOD 05162002962479 ver 1. 0 1 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Training Agenda • System overview • MULTISTIX® 10 SG Overview • Performing testing: ü Quality control testing ü Patient testing • Performing maintenance • Ordering information 2 Maria Peluso-Lapsley| CDM Marketing Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

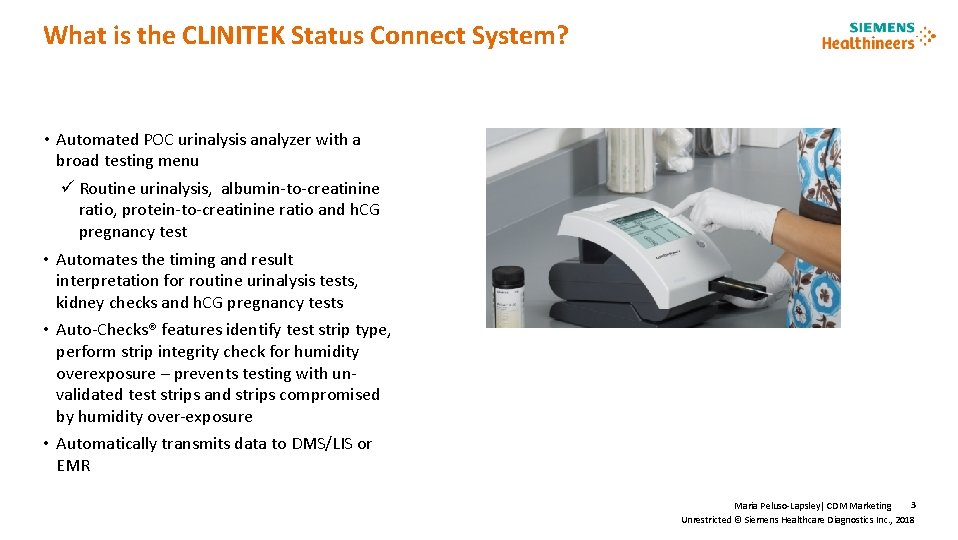
What is the CLINITEK Status Connect System? • Automated POC urinalysis analyzer with a broad testing menu ü Routine urinalysis, albumin-to-creatinine ratio, protein-to-creatinine ratio and h. CG pregnancy test • Automates the timing and result interpretation for routine urinalysis tests, kidney checks and h. CG pregnancy tests • Auto-Checks® features identify test strip type, perform strip integrity check for humidity overexposure – prevents testing with unvalidated test strips and strips compromised by humidity over-exposure • Automatically transmits data to DMS/LIS or EMR 3 Maria Peluso-Lapsley| CDM Marketing Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

System Overview Click to add footnote second line 4 Unrestricted Restricted © Siemens Healthcare Diagnostics Inc. , 2018

CLINITEK Status Connect System Overview The CLINITEK Status® Connect System is a portable, easy to use analyzer. It is designed to read only Siemens Healthineers Urinalysis test strips and Clinitest® h. CG tests. • Measures the following in urine: Albumin, Bilirubin, Blood (Occult), Creatinine, Glucose, Ketone, Leukocytes, Nitrite, p. H, Protein-to-Creatinine Ratio, Albumin-to-Creatinine Ratio, Specific Gravity, Urobilinogen, and human Chorionic Gonadotropin (h. CG) • These measurements are used to assist diagnosis in the following areas: Kidney function, Urinary tract infections, Metabolic disorders (such as diabetes mellitus), Liver function, and Pregnancy 5 Maria Peluso-Lapsley| CDM Marketing Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Setting up the Analyzer Click to add footnote second line 6 Unrestricted Restricted © Siemens Healthcare Diagnostics Inc. , 2018

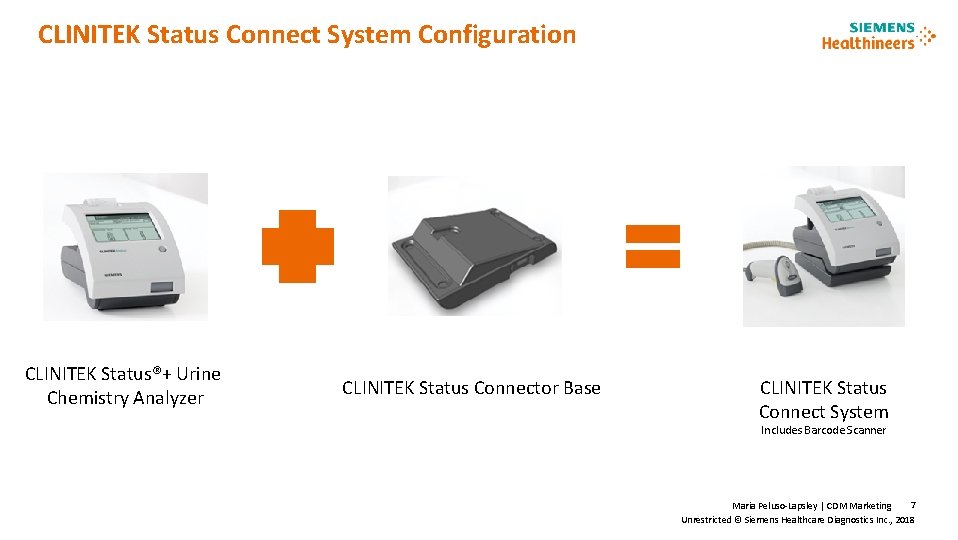
CLINITEK Status Connect System Configuration CLINITEK Status®+ Urine Chemistry Analyzer CLINITEK Status Connector Base CLINITEK Status Connect System Includes Barcode Scanner 7 Maria Peluso-Lapsley | CDM Marketing Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

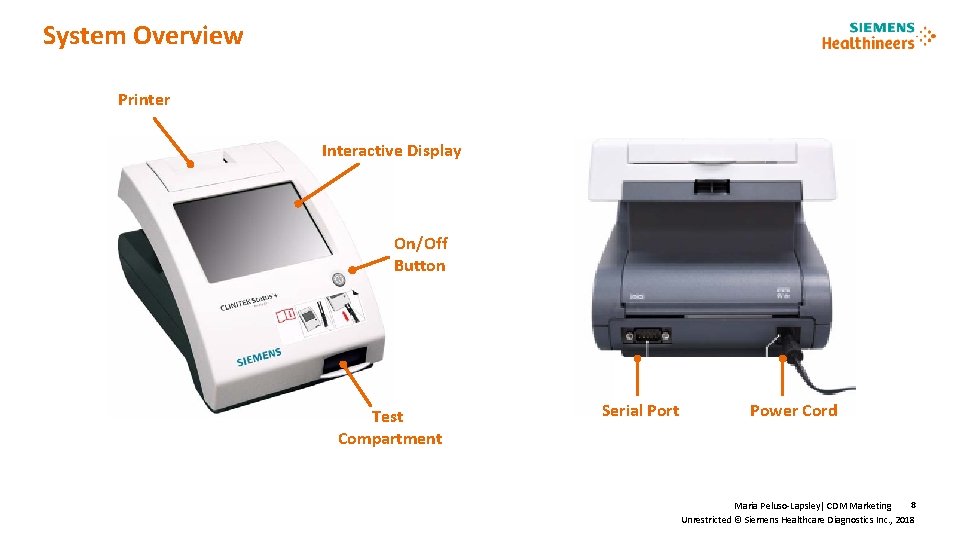
System Overview Printer Interactive Display On/Off Button Test Compartment Serial Port Power Cord 8 Maria Peluso-Lapsley| CDM Marketing Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

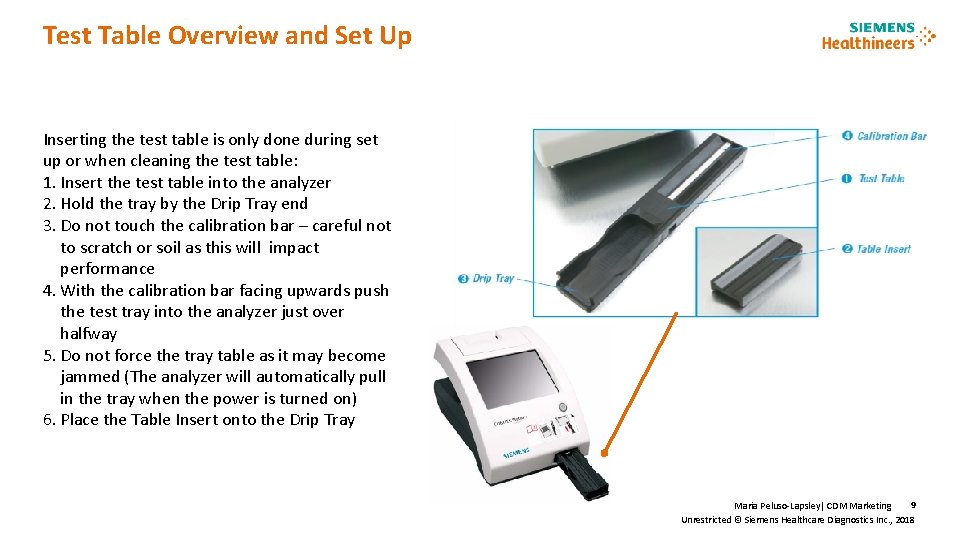
Test Table Overview and Set Up Inserting the test table is only done during set up or when cleaning the test table: 1. Insert the test table into the analyzer 2. Hold the tray by the Drip Tray end 3. Do not touch the calibration bar – careful not to scratch or soil as this will impact performance 4. With the calibration bar facing upwards push the test tray into the analyzer just over halfway 5. Do not force the tray table as it may become jammed (The analyzer will automatically pull in the tray when the power is turned on) 6. Place the Table Insert onto the Drip Tray 9 Maria Peluso-Lapsley| CDM Marketing Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

CLINITEK Status Connector Overview • Adding the connector platform provides the following: ü Barcode scanning for entry of patient and operator IDs, test strip, cassette and QC material lot number and expiration dating ü Interfacing to data management systems and network via Ethernet ü Bi-directional functionality such as operator ID downloading and remote QC lock out ü QC mode for QC lock out and separate database for QC testing data ü Wireless capability (if site access points are compatible) 5 1. Short DC power cable 2. Optional wireless adapter 3. Serial (RS-232) cable 4. Power supply adaptor 5. Optional barcode scanner Maria Peluso-Lapsley| CDM Marketing 10 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Loading the Printer Paper To load thermal printer paper or label roll, perform the following steps: 1. 2. 3. 4. 5. Turn the back of the analyzer to face you Pull on the tab to open the printer cover Open the paper roll compartment Lift the paper holding arm into the open, upright position Insert a new paper roll it should unroll from underneath and roll toward the compartment wall 6. Feed the paper up along the wall and through the printer until 4 inches of paper feeds through 7. Feed the edge of the paper through the printer cover 8. Push the paper holding arm down in the closed position (if this step is missed the printer will not print) 9. Close both covers by clicking into place 1 Paper holding arm 2 Printer paper Maria Peluso-Lapsley| CDM Marketing 11 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Powering Analyzer On/Off If you power on the analyzer for the first time, the Start Up Wizard will guide the set-up procedure: 1. Press the on/off button on the front of the analyzer • Analyzer performs automatic checks when powered on To power off the analyzer, perform the following steps: 1. Ensure that no strip or cassette is on the test table and that the table and insert are clean 2. Hold the on/off button down for at least 2 seconds 3. Analyzer pulls in the test table and will turn off. • If the test table hasn’t been cleared of test strip or cassette, it will be pushed out by the analyzer and powered off • To power off, the analyzer must be powered back on, and the test strips or cassette must be cleared Maria Peluso-Lapsley| CDM Marketing 12 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Customize Set-up • Select the testing mode that best fits your site needs. • There are three modes to select from: ü Quick test – does not require any patient operator data to be entered ü Full test – requires operator, patient and other fixed data to be entered ü Custom Path: Select Instrument Set Up > Operator and Patient Information Maria Peluso-Lapsley| CDM Marketing 13 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Customize Set-up • Instrument set up allows for customization and standardization of running your urinalysis program. • Review each area to select your settings: ü Results format – units and flagging ü Connectivity – define connectivity settings ü Urinalysis test setting – handling of lot and expiration dating ü Authorized operators – to set up operator access and lock out ü Printer settings – define printing requirements ü QC settings – define QC testing needs For more detailed instructions, refer to the operator manual or analyzer in-service training tool. Path: Select Instrument Set Up > Instrument Settings Maria Peluso-Lapsley| CDM Marketing 14 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

MULTISTIX 10 SG Overview Click to add footnote second line 15 Unrestricted Restricted © Siemens Healthcare Diagnostics Inc. , 2018

MULTISTIX® 10 SG Test Overview • For professional in vitro diagnostic use in near patient (point of care) and centralized laboratory locations • The strips are intended for use in at-risk patient groups to assist diagnosis in the following areas: ü kidney function, urinary tract infections, carbohydrate metabolism (e. g. , diabetes mellitus), liver function ü measure physical characteristics, including acid-base • For use on CLINITEK Status® family of analyzers • Results reported in 1 minute • CLIA-waived when tested on the CLINITEK Status® family of analyzers For measuring protein, blood, leukocytes, nitrite, glucose, ketone (acetoacetic acid), p. H, specific gravity, bilirubin and urobilinogen. Maria Peluso-Lapsley| CDM Marketing 16 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

MULTISTIX 10 SG Configuration • Urine test strip bottle: ü 100 test strips ü desiccant to prevent moisture • Instructions for use can be downloaded from the internet Maria Peluso-Lapsley| CDM Marketing 17 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Test Bottle Storage and Stability • All unused strips must remain in the original bottle. • Transfer to any other container may cause reagent strips to deteriorate and become unreactive • Store at temperatures between 15– 30°C (59– 86°F) • Do not use the strips after their expiration date (Note: analyzer can be programmed to prevent use of expired test strips) • Do not store the bottle in direct sunlight and do not remove the desiccant from the bottle Maria Peluso-Lapsley| CDM Marketing 18 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Getting Ready for Testing Click to add footnote second line 19 Unrestricted Restricted © Siemens Healthcare Diagnostics Inc. , 2018

Supplies Needed to Conduct Testing Analyzer and Tests (supplied by Siemens Healthineers or authorized distributor): CLINITEK Status Family of Analyzers MULTISTIX 10 SG Test Strips Chek-Stix Quality Control Materials Recommended Supplies: (non Siemens Healthineers items): Urine specimen collection containers Maria Peluso-Lapsley | CDM Marketing 20 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Performing Quality Control Testing Click to add footnote second line 21 Unrestricted Restricted © Siemens Healthcare Diagnostics Inc. , 2018

Quality Control Testing Modes CLINITEK Status+ Analyzer (stand-alone): No QC mode. Control samples are treated and stored as patient samples. User must enter control name or ID to distinguish results Control results must be manually compared to the values assigned by the control manufacturer If the control results are not favorable, do not test patient samples until quality control is passed CLINITEK Status Connect System (with connector base): Has a separate QC mode and database for QC results CLINITEK Status® Connect Systems can be set-up by user to automatically check QC results and initiate a QC lock out when results fail If the control results are not favorable, do not test patient samples until quality control is passed Maria Peluso-Lapsley| CDM Marketing 22 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Quality Control Testing Recommendations 1. Test positive and negative quality controls with new lots, new shipments of reagents, and when you open a new bottle of reagent strips 2. Test reagents monthly that are stored for more than 30 days 3. Run QC tests to ensure reagent strips integrity; train new users; confirm test performance; and when patients’ clinical conditions or symptoms do not match 4. Run QC tests per your laboratory procedures 5. Liquid ready-to-use controls are available 6. Do not use water as a negative control Maria Peluso-Lapsley| CDM Marketing 23 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

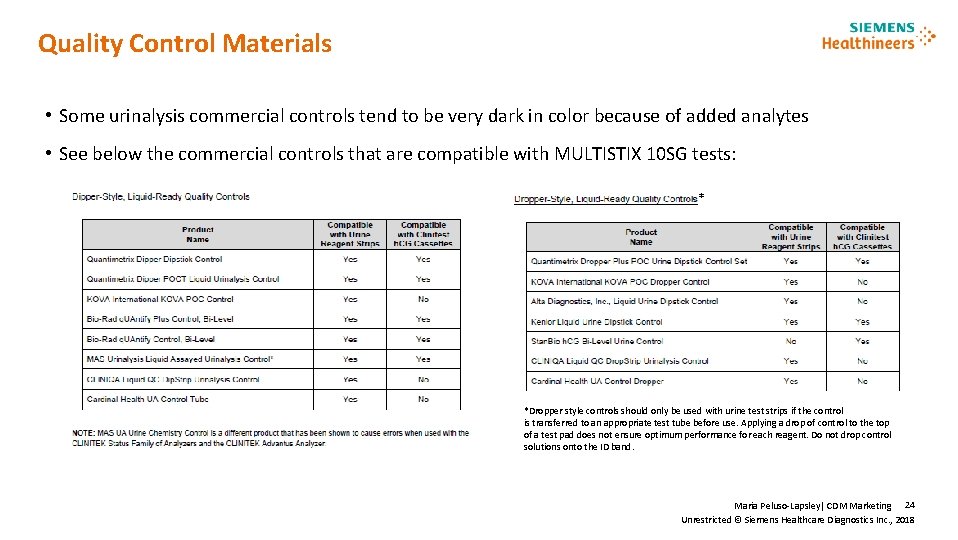
Quality Control Materials • Some urinalysis commercial controls tend to be very dark in color because of added analytes • See below the commercial controls that are compatible with MULTISTIX 10 SG tests: * *Dropper style controls should only be used with urine test strips if the control is transferred to an appropriate test tube before use. Applying a drop of control to the top of a test pad does not ensure optimum performance for each reagent. Do not drop control solutions onto the ID band. Maria Peluso-Lapsley| CDM Marketing 24 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Chek-Stix Storage and Stability Reconstitution Stability • Reconstituted controls are stable for 8 hours at 18– 23°C (64– 73°F) except for bilirubin in the Positive Control, which is stable for 3 hours • Store Chek-Stix® Control Strips in the original, tightly capped bottle at temperatures between 15– 30°C • Do not store the bottle in direct sunlight. • Do not remove the desiccant from bottle • If your laboratory operates outside of this temperature range, reconstituted solution should be refrigerated at 2– 8°C (34– 46°F) to maintain the 8 hour stability • Allow refrigerated control solutions to equilibrate to ambient temperature prior to use • Control strips are stable until the expiration date shown on the bottle label Maria Peluso-Lapsley| CDM Marketing 25 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Prepare Chek-Stix Quality Controls Directions: 1. Place 12 m. L of distilled or deionized water in an appropriately labeled specimen tube. Do not use tap water. 2. Remove a Chek-Stix Control Strip from the bottle and replace the cap immediately and tightly. 3. Place the strip into the tube. Cap tightly. 4. Repeat Steps 1– 3 if using a second control. 5. Gently invert the tube(s) back and forth for 2 minutes. 6. Allow the tube(s) to stand for 30 minutes at room temperature. 7. Invert one more time, then remove and discard the strip(s), according to your standard laboratory procedures. Maria Peluso-Lapsley| CDM Marketing 26 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

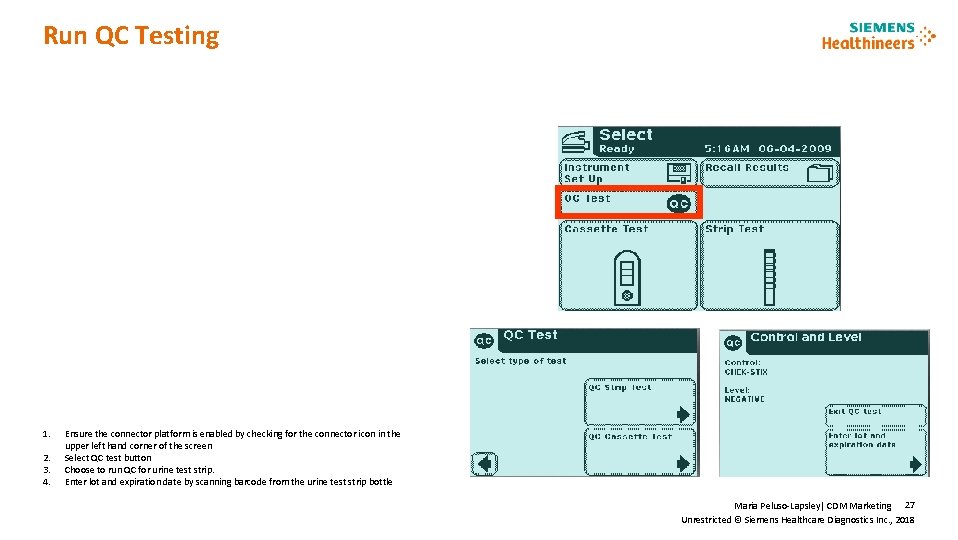
Run QC Testing 1. 2. 3. 4. New Product Features Ensure the connector platform is enabled by checking for the connector icon in the upper left hand corner of the screen Select QC test button Choose to run QC for urine test strip. Enter lot and expiration date by scanning barcode from the urine test strip bottle Maria Peluso-Lapsley| CDM Marketing 27 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

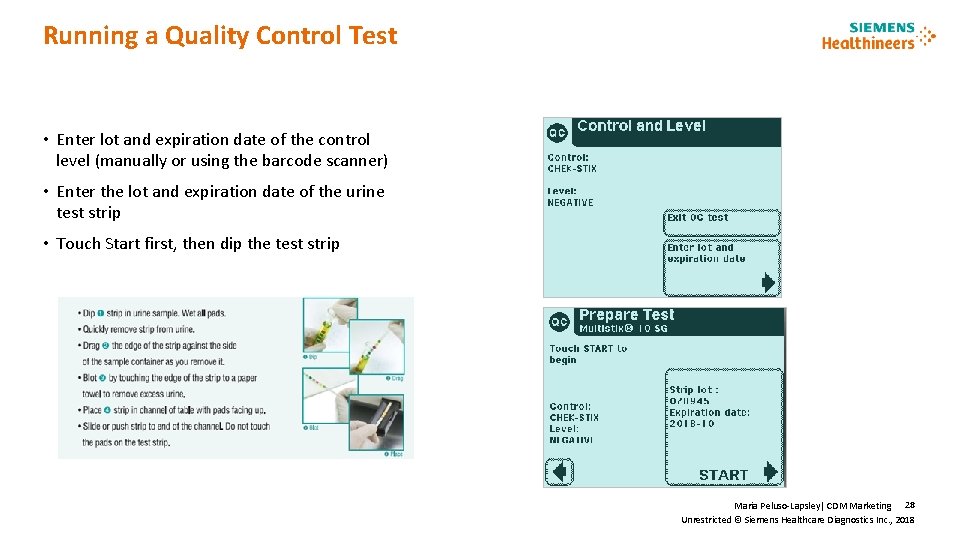
Running a Quality Control Test • Enter lot and expiration date of the control level (manually or using the barcode scanner) • Enter the lot and expiration date of the urine test strip • Touch Start first, then dip the test strip Maria Peluso-Lapsley| CDM Marketing 28 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Performing Patient Testing Click to add footnote second line 29 Unrestricted Restricted © Siemens Healthcare Diagnostics Inc. , 2018

Specimen Collection and Handling • • Collect urine into a clean, dry container First morning or random collections are acceptable Test fresh urine samples within 2 hours of collection If cannot be tested within 2 hours, refrigerate specimens at 2° to 8°C (36° to 46°F) for up to 72 hours, if the testing is not performed immediately • If samples are refrigerated, bring them to room temperature 15° to 30°C (59° to 86°F) before testing • The use of urine preservatives is not recommended • Do not use urine that looks bloody or is not a normal color Maria Peluso-Lapsley| CDM Marketing 30 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Performing a Test Maria Peluso-Lapsley| CDM Marketing 31 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

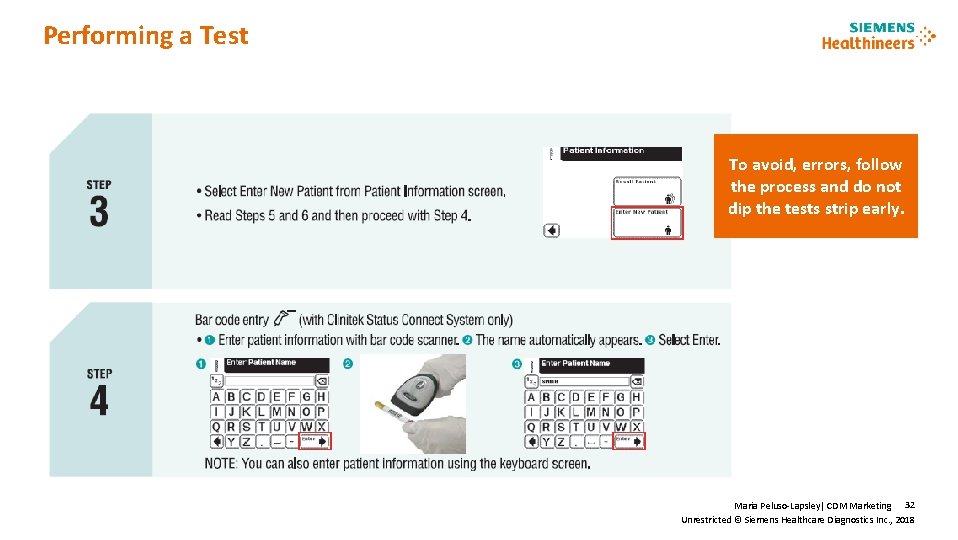
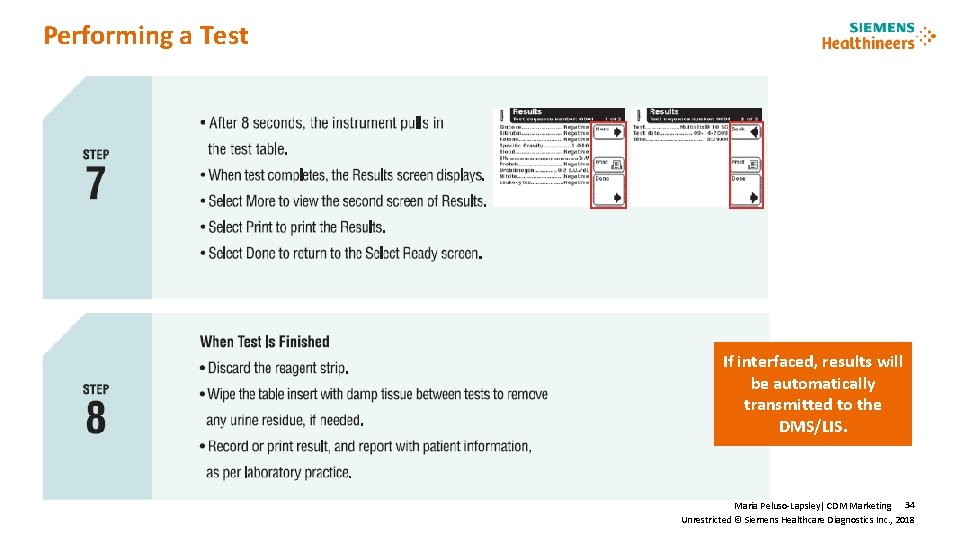
Performing a Test To avoid, errors, follow the process and do not dip the tests strip early. Maria Peluso-Lapsley| CDM Marketing 32 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

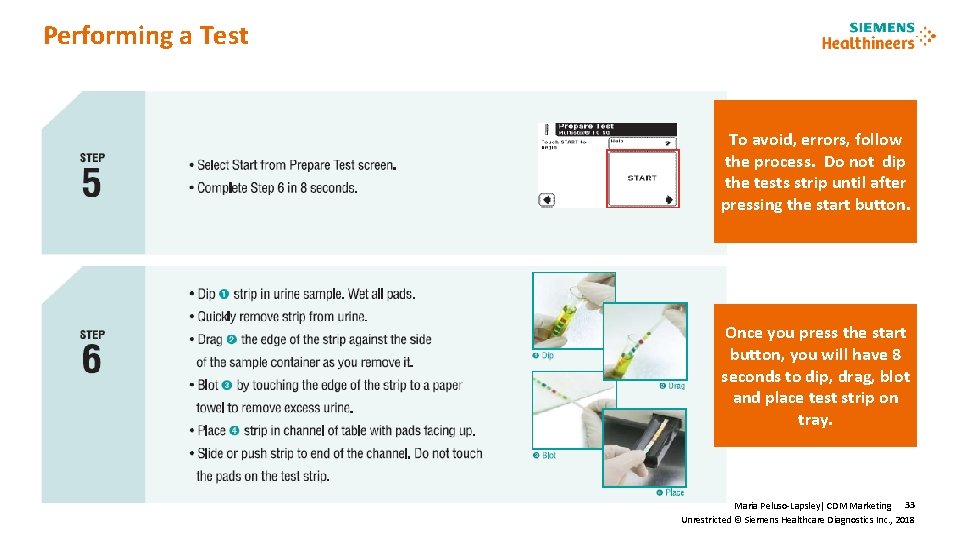
Performing a Test To avoid, errors, follow the process. Do not dip the tests strip until after pressing the start button. Once you press the start button, you will have 8 seconds to dip, drag, blot and place test strip on tray. Maria Peluso-Lapsley| CDM Marketing 33 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Performing a Test If interfaced, results will be automatically transmitted to the DMS/LIS. Maria Peluso-Lapsley| CDM Marketing 34 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

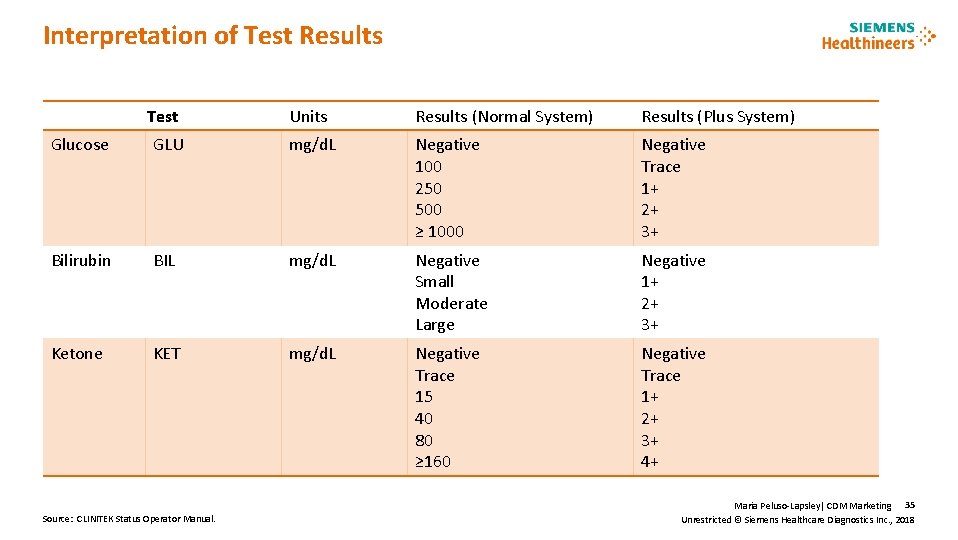
Interpretation of Test Results Test Units Results (Normal System) Results (Plus System) Glucose GLU mg/d. L Negative 100 250 500 ≥ 1000 Negative Trace 1+ 2+ 3+ Bilirubin BIL mg/d. L Negative Small Moderate Large Negative 1+ 2+ 3+ Ketone KET mg/d. L Negative Trace 15 40 80 ≥ 160 Negative Trace 1+ 2+ 3+ 4+ Source: CLINITEK Status Operator Manual. Maria Peluso-Lapsley| CDM Marketing 35 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

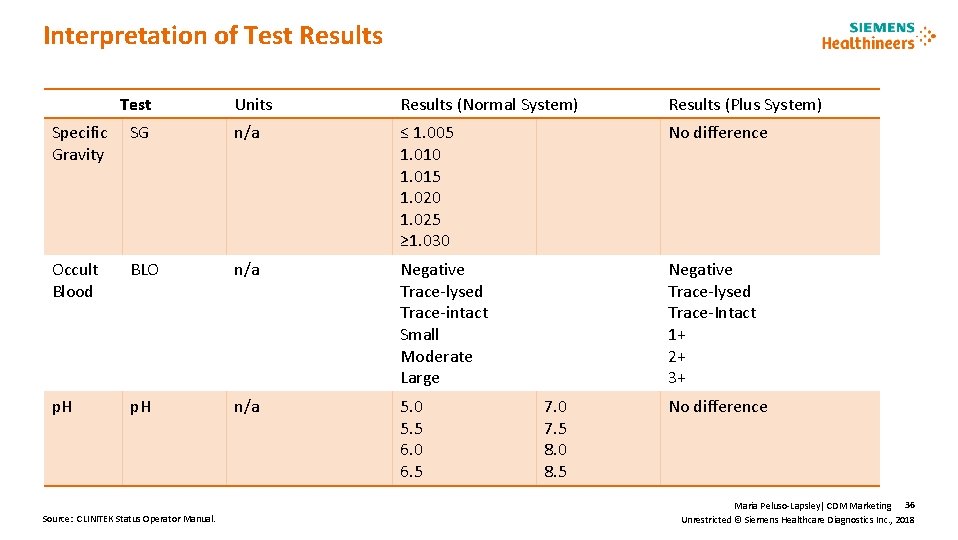
Interpretation of Test Results Test Units Results (Normal System) Results (Plus System) Specific Gravity SG n/a ≤ 1. 005 1. 010 1. 015 1. 020 1. 025 ≥ 1. 030 No difference Occult Blood BLO n/a Negative Trace-lysed Trace-intact Small Moderate Large Negative Trace-lysed Trace-Intact 1+ 2+ 3+ p. H n/a 5. 0 5. 5 6. 0 6. 5 Source: CLINITEK Status Operator Manual. 7. 0 7. 5 8. 0 8. 5 No difference Maria Peluso-Lapsley| CDM Marketing 36 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

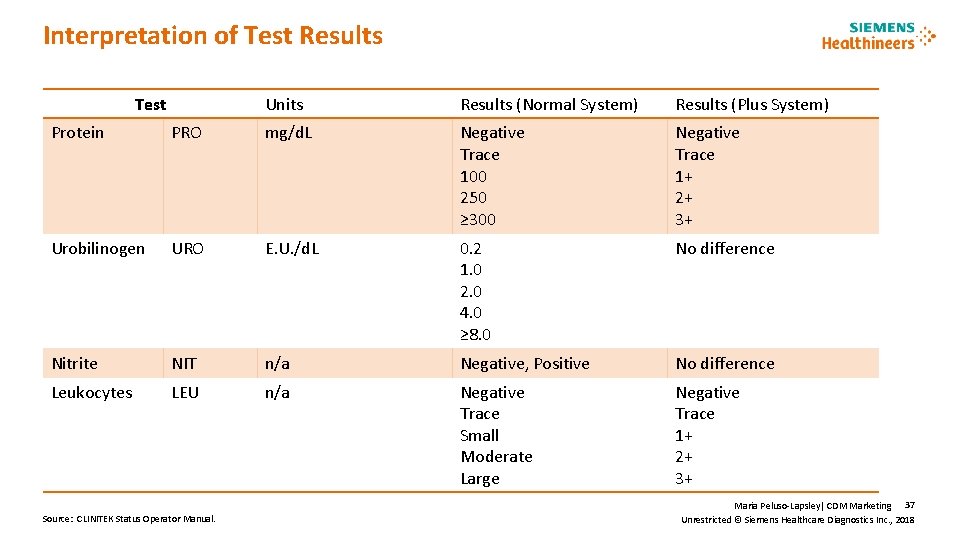
Interpretation of Test Results Test Units Results (Normal System) Results (Plus System) Protein PRO mg/d. L Negative Trace 100 250 ≥ 300 Negative Trace 1+ 2+ 3+ Urobilinogen URO E. U. /d. L 0. 2 1. 0 2. 0 4. 0 ≥ 8. 0 No difference Nitrite NIT n/a Negative, Positive No difference Leukocytes LEU n/a Negative Trace Small Moderate Large Negative Trace 1+ 2+ 3+ Source: CLINITEK Status Operator Manual. Maria Peluso-Lapsley| CDM Marketing 37 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Avoiding Errors • Do not remove the strip from the bottle until immediately before it is to be used for testing • Replace the cap immediately and tightly after removing the reagent strip • Discoloration or darkening of the test pads may indicate deterioration: 1. Auto-Checks technology will detect overexposure to humidity for urine test strips and prevent patient testing. 2. Analyzer automatically detects if test strips, cassette materials or QC materials are expired and prevent patient testing. • Contamination of the urine specimen with skin cleansers containing chlorhexidine may affect protein (and to a lesser extent specific gravity and bilirubin) test results • The user should determine whether the use of such skin cleansers is warranted • It is especially important to use fresh urine to obtain optimal results with the tests for bilirubin and urobilinogen, as these compounds are very unstable when exposed to room temperature and light • Do not touch the test areas of the strip Maria Peluso-Lapsley| CDM Marketing 38 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Performing Routine Maintenance/ Cleaning Click to add footnote second line 39 Unrestricted Restricted © Siemens Healthcare Diagnostics Inc. , 2018

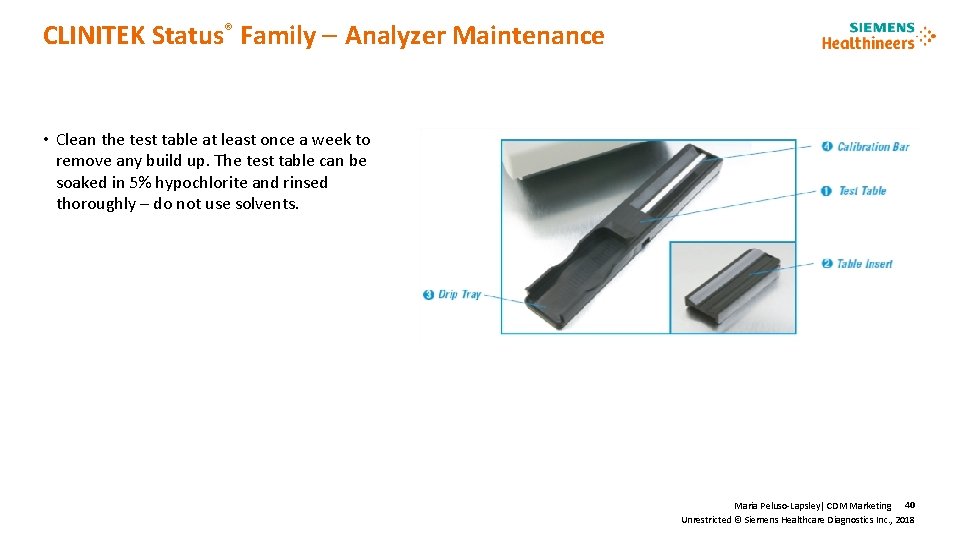
CLINITEK Status® Family Analyzer Maintenance • Clean the test table at least once a week to remove any build up. The test table can be soaked in 5% hypochlorite and rinsed thoroughly – do not use solvents. Maria Peluso-Lapsley| CDM Marketing 40 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

CLINITEK Status Family - Analyzer Maintenance • Check that the calibration strip on the instrument is clean every day. If not, clean with a cotton swab or soft cloth and water only, ensuring that you do not scratch it. Maria Peluso-Lapsley| CDM Marketing 41 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Replacing the Printer Paper Maria Peluso-Lapsley| CDM Marketing 42 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Ordering Information Click to add footnote second line 43 Unrestricted Restricted © Siemens Healthcare Diagnostics Inc. , 2018

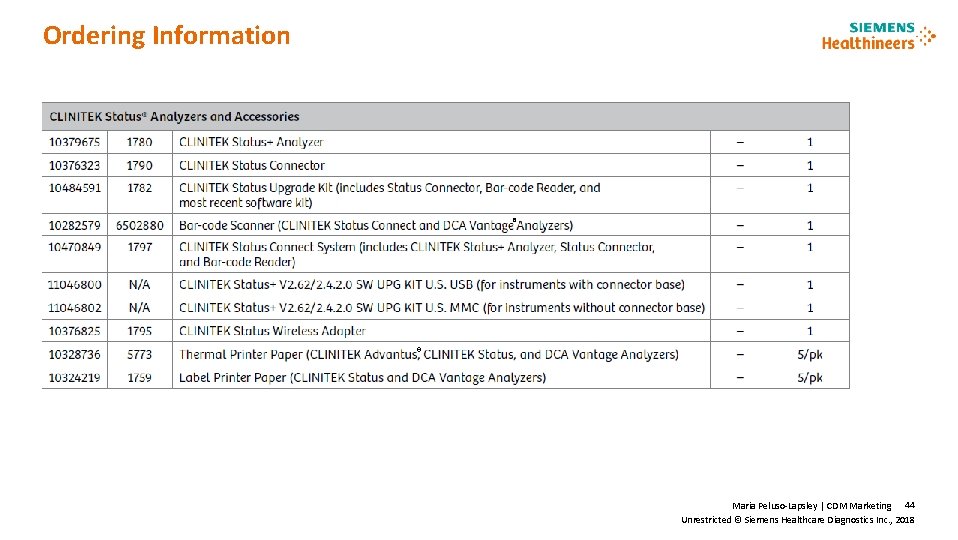
Ordering Information ® ® Maria Peluso-Lapsley | CDM Marketing 44 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

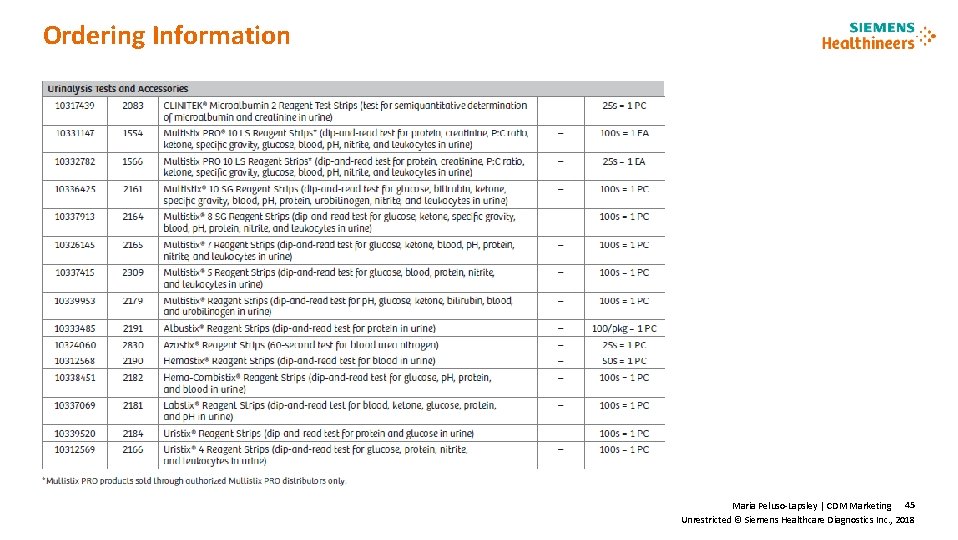
Ordering Information Maria Peluso-Lapsley | CDM Marketing 45 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018

Thank you! CLINITEK, CLINITEK Status, MULTISTIX, CLINITEST, Chek-Stix, Auto-Checks, CLINITEK Advantus, DCA Vantage, MULTISTIX PRO, Albustix, Azostix, Hema. Combistix, Labstix, Uristix and all associated marks are trademarks of Siemens Healthcare Diagnostics Inc. , or its affiliates. All other trademarks and brands are the property of their respective owners. Maria Peluso-Lapsley| CDM Marketing 46 Unrestricted © Siemens Healthcare Diagnostics Inc. , 2018
 Clinitek status connect system
Clinitek status connect system Inservice
Inservice Inservice examples
Inservice examples Inservice training assignment
Inservice training assignment Grad connect application
Grad connect application Shop stream connect
Shop stream connect Trimble enterprise community
Trimble enterprise community Expedia quick connect
Expedia quick connect Leap connect practice test
Leap connect practice test Lser connect ecom
Lser connect ecom Borders divide customs connect
Borders divide customs connect Everyone communicates few connect quotes
Everyone communicates few connect quotes Masteryconnect.com
Masteryconnect.com Oasys omgeo
Oasys omgeo Mcts tutorial
Mcts tutorial Netflix oca
Netflix oca Control active x
Control active x Nopcommerce dynamics ax connector
Nopcommerce dynamics ax connector Detailing connect
Detailing connect Pnpx device association
Pnpx device association Adobe connect update
Adobe connect update Vishal patel novartis
Vishal patel novartis Anthem hoosier care connect
Anthem hoosier care connect Eops mt sac
Eops mt sac Atrezzo kepro
Atrezzo kepro Testout network
Testout network Mastery connect
Mastery connect Rrt connect
Rrt connect Connect four history
Connect four history Sasc connect
Sasc connect Nail contraindications
Nail contraindications France connect inconvénients
France connect inconvénients Campus connect ivy tech
Campus connect ivy tech Salesforce files connect implementation guide
Salesforce files connect implementation guide Connect student registration
Connect student registration L connect
L connect Monkey see monkey do monkey connect
Monkey see monkey do monkey connect Alitalia business connect
Alitalia business connect Fidelity connect
Fidelity connect Brownie presentation
Brownie presentation Invoice ge
Invoice ge Usg one connect
Usg one connect Farmers may someday clone
Farmers may someday clone Dell secure connect gateway
Dell secure connect gateway What is the maximum number of pieces
What is the maximum number of pieces Appetize pricing
Appetize pricing Logi
Logi