Multiplex immunoassay MIA based on x MAP Luminex
Multiplex immunoassay (MIA) based on x. MAP© (Luminex) Technology Measles Ig. G performance indicators RIVM Centre for Infectious Disease Control The Netherlands Luminex Technology x. MAP® 1 Rob van Binnendijk Fiona van der Klis
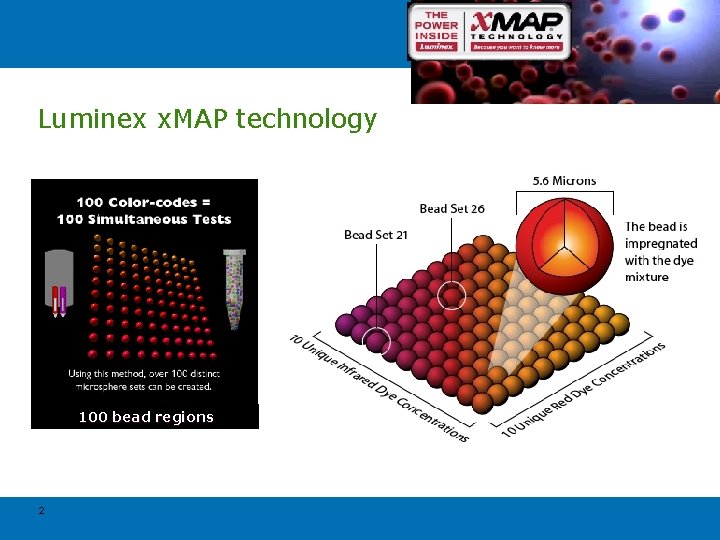
Luminex x. MAP technology 100 bead regions 2
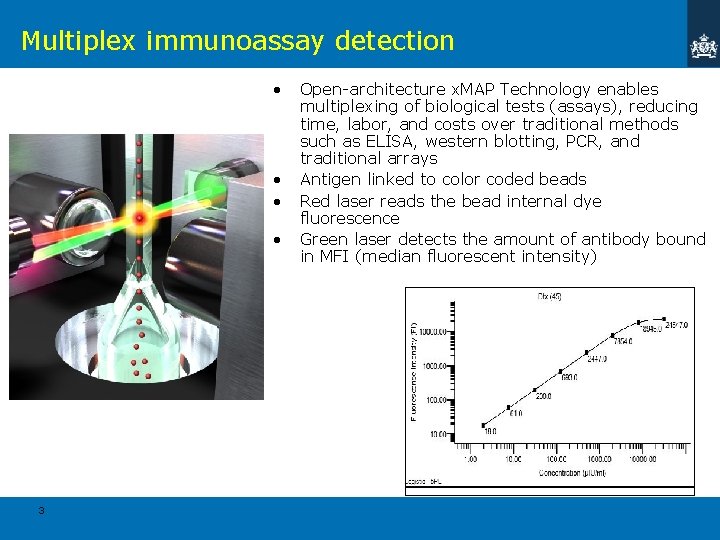
Multiplex immunoassay detection • • 3 Open-architecture x. MAP Technology enables multiplexing of biological tests (assays), reducing time, labor, and costs over traditional methods such as ELISA, western blotting, PCR, and traditional arrays Antigen linked to color coded beads Red laser reads the bead internal dye fluorescence Green laser detects the amount of antibody bound in MFI (median fluorescent intensity)
Application: Immune Surveillance ● vaccine coverage (Ig. G seropositives) ● immunity in the population: correlate of protection (Co. P) > Detect gaps in immunity, groups/populations at risk 4

Multiplex Immuno Assay (MIA) : Ig. G quantitatively bead + measles ag (purified Edmonston, RIVM grade) Ig. G in sample detection (anti-Ig. G RPE) bead + mumps ag (Jeryl Lynn) 5 bead + rubella ag (HPV-77)
Edmonston virus produced on Vero cells (bioreactor) concentrated by ultra filtration (1000 k. D) ultracentrifugation > disc. 20/40/70 sucrose gradient 40/70 virus fraction freeze-dried (1 -5 mg/ml) 6
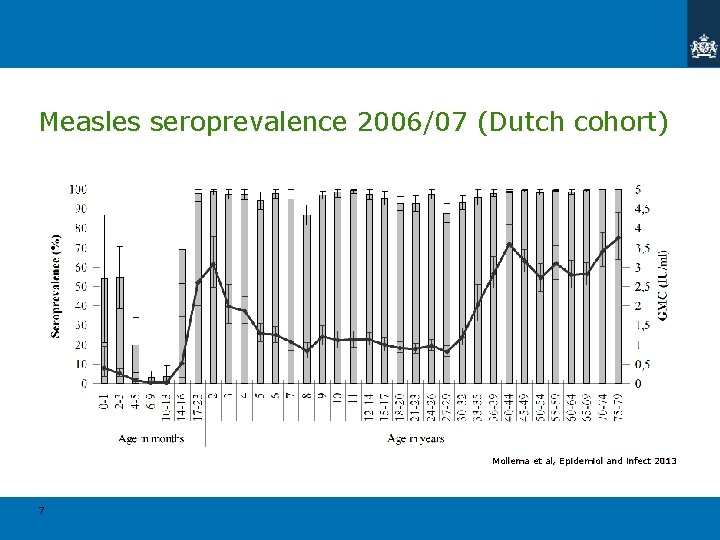
Measles seroprevalence 2006/07 (Dutch cohort) Mollema et al, Epidemiol and infect 2013 7
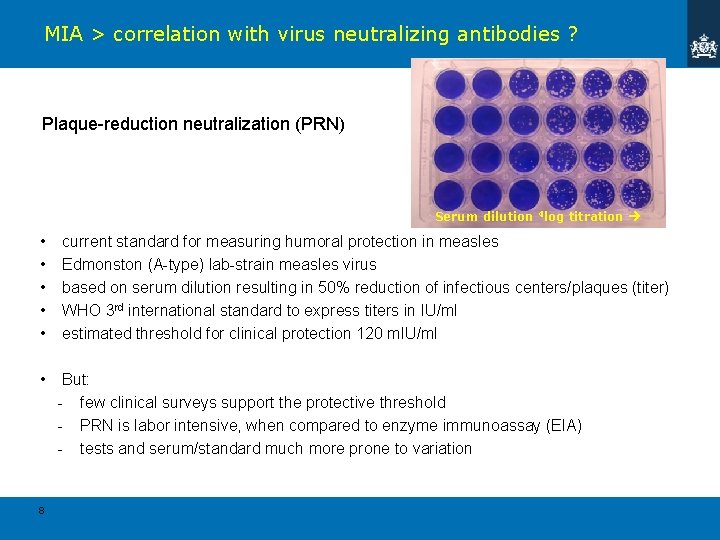
MIA > correlation with virus neutralizing antibodies ? Plaque-reduction neutralization (PRN) Serum dilution 4 log titration • • • current standard for measuring humoral protection in measles Edmonston (A-type) lab-strain measles virus based on serum dilution resulting in 50% reduction of infectious centers/plaques (titer) WHO 3 rd international standard to express titers in IU/ml estimated threshold for clinical protection 120 m. IU/ml • But: - few clinical surveys support the protective threshold - PRN is labor intensive, when compared to enzyme immunoassay (EIA) - tests and serum/standard much more prone to variation 8

MIA > correlation with glycoprotein-specific assays ? Measles F-/H- transfected cell-lines (de Swart et al. 1998) - serum antibody titrations - staining with FITC-conjugated anti-human Ig. G - FACS fluorescence analysis - sensitivity > EIA (Hartter et al. 2000) * * mel/Ju. So 9 mel/Ju. So F mel/Ju. So H
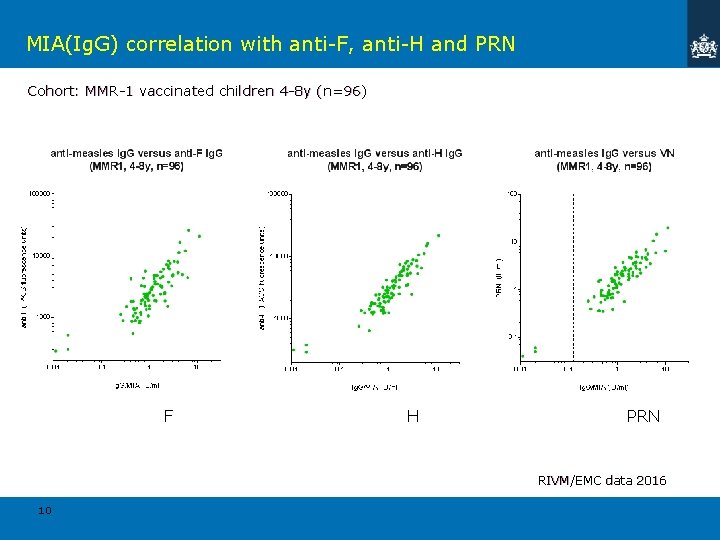
MIA(Ig. G) correlation with anti-F, anti-H and PRN Cohort: MMR-1 vaccinated children 4 -8 y (n=96) F H PRN RIVM/EMC data 2016 10
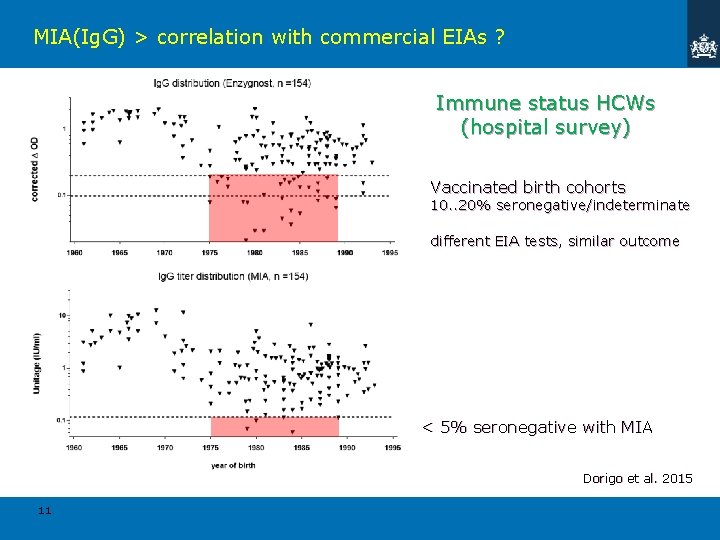
MIA(Ig. G) > correlation with commercial EIAs ? Immune status HCWs (hospital survey) Vaccinated birth cohorts 10. . 20% seronegative/indeterminate different EIA tests, similar outcome < 5% seronegative with MIA Dorigo et al. 2015 11
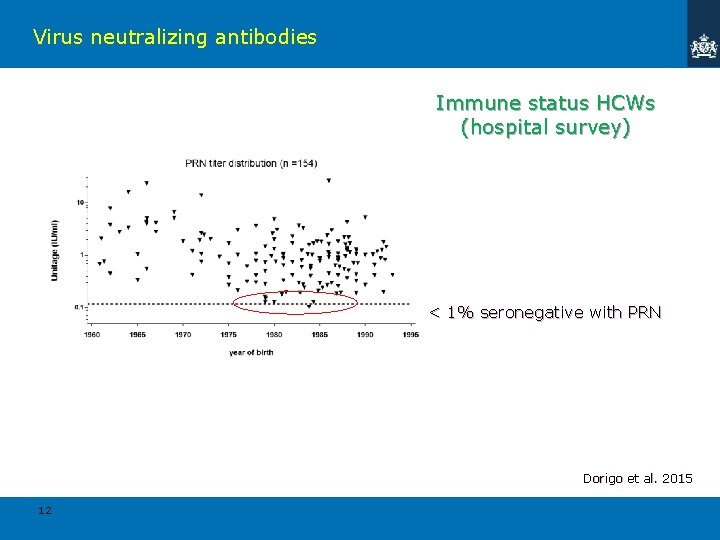
Virus neutralizing antibodies Immune status HCWs (hospital survey) < 1% seronegative with PRN Dorigo et al. 2015 12
measles serostatus: notes and challenges ● Population-wise, majority of vaccinated persons have neutralizing antibodies ● Commercial EIA tests lack sensitivity, to detect protective antibodies in vaccinated persons (>10%) ● Better correlation between PRN and MIA (Luminex), not absolute Challenges: ● Alternative serological tests/indicators for serological comparisons ● Investigate different vaccine lots/regimens/programmes ● Testing of low-titre sera and seronegatives (LLOQ) ● Improve PRN testing >development high-throughput (96 wells/FRN) format 13
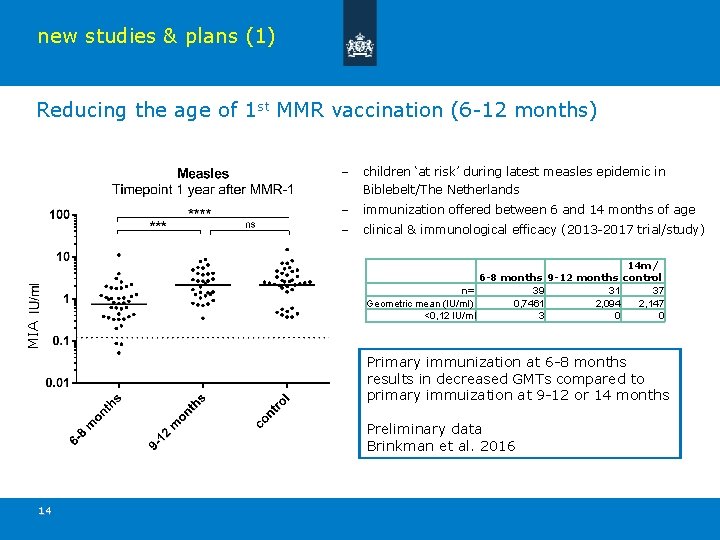
new studies & plans (1) Reducing the age of 1 st MMR vaccination (6 -12 months) – children ‘at risk’ during latest measles epidemic in Biblebelt/The Netherlands – immunization offered between 6 and 14 months of age – clinical & immunological efficacy (2013 -2017 trial/study) MIA 14 m/ 6 -8 months 9 -12 months control 39 31 37 n= Geometric mean (IU/ml) 0, 7461 2, 094 2, 147 <0, 12 IU/ml 3 0 0 Primary immunization at 6 -8 months results in decreased GMTs compared to primary immuization at 9 -12 or 14 months Preliminary data Brinkman et al. 2016 14
new studies & plans (2) ● New large population-based survey in The Netherlands 2016 (Pienter-3) ● Systematic analysis of Ig. G (MIA) and PRN in immunized persons from different vaccinate regimes/programs/lots ● Extend analysis to heterologous (wildtype) measles strains (EMC/RIVM) ● Serving Lab. Net with high-throughput Luminex-based MMR serology ● Capacity building for multiplex (MIA, FRNT) based serology for measles and mumps (EMC/RIVM) 15

Acknowledgements Fiona van der Klis Gaby Smits Iris Brinkman Nynke Rots Jeroen Kerkhof Hinke ten Hulscher RIVM/Virology & Immunology Lab Bilthoven, The Netherlands Rik de Swart Virosciences/EMC Rotterdam, The Netherlands 16
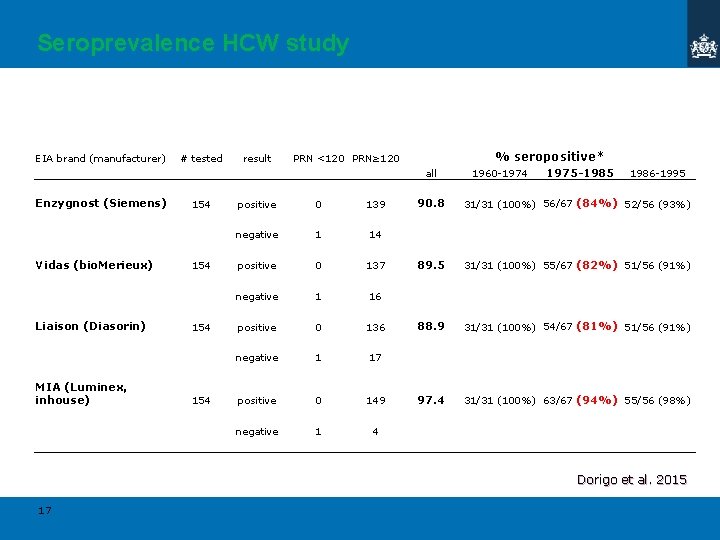
Seroprevalence HCW study EIA brand (manufacturer) # tested result % seropositive* PRN <120 PRN≥ 120 all Enzygnost (Siemens) Vidas (bio. Merieux) Liaison (Diasorin) MIA (Luminex, inhouse) 154 154 positive 0 139 negative 1 14 positive 0 137 negative 1 16 positive 0 136 negative 1 17 positive 0 149 negative 1 4 1960 -1974 1975 -1985 1986 -1995 90. 8 31/31 (100%) 56/67 (84%) 52/56 (93%) 89. 5 31/31 (100%) 55/67 (82%) 51/56 (91%) 88. 9 31/31 (100%) 54/67 (81%) 51/56 (91%) 97. 4 31/31 (100%) 63/67 (94%) 55/56 (98%) Dorigo et al. 2015 17
- Slides: 17