MULTIPLE REACTIONS Lecturer Dr Hairul Nazirah Abdul Halim
MULTIPLE REACTIONS Lecturer: Dr. Hairul Nazirah Abdul Halim

� Introduction 1. Types of Reactions 2. Selectivity & Yield � Maximizing the Desired Product 1. Parallel Reactions - for one reactant - for two reactants 2. Series Reactions � Algorithm for solving Reaction Eng. Problems
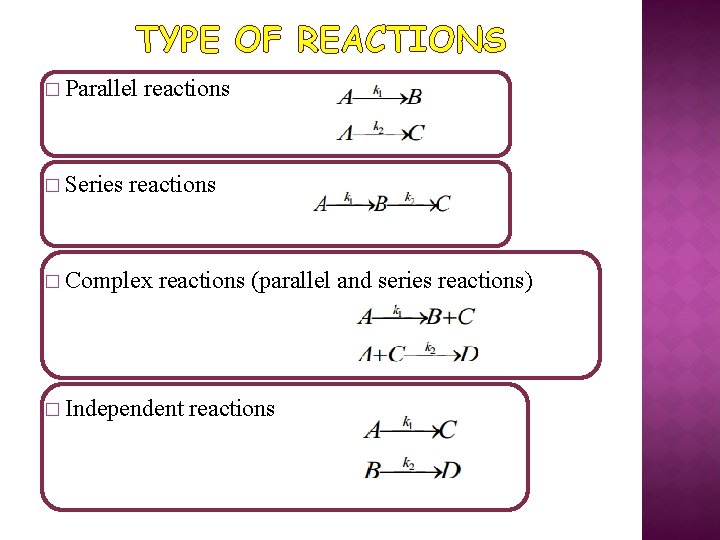
TYPE OF REACTIONS � Parallel � Series reactions � Complex reactions (parallel and series reactions) � Independent reactions
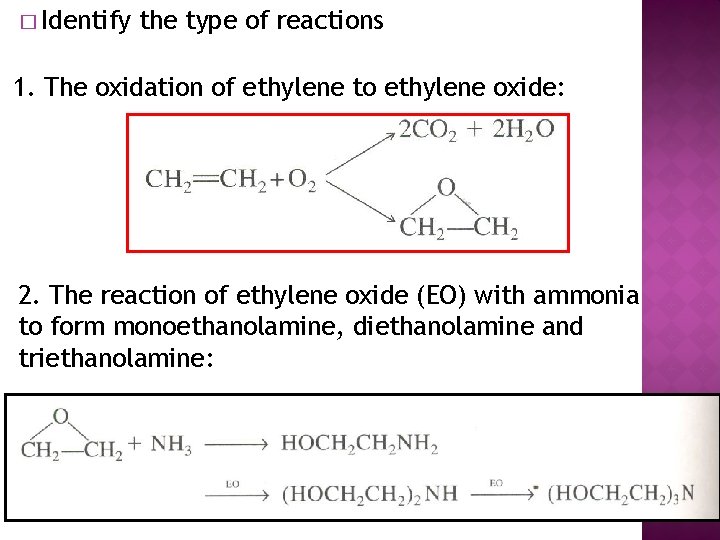
� Identify the type of reactions 1. The oxidation of ethylene to ethylene oxide: 2. The reaction of ethylene oxide (EO) with ammonia to form monoethanolamine, diethanolamine and triethanolamine:
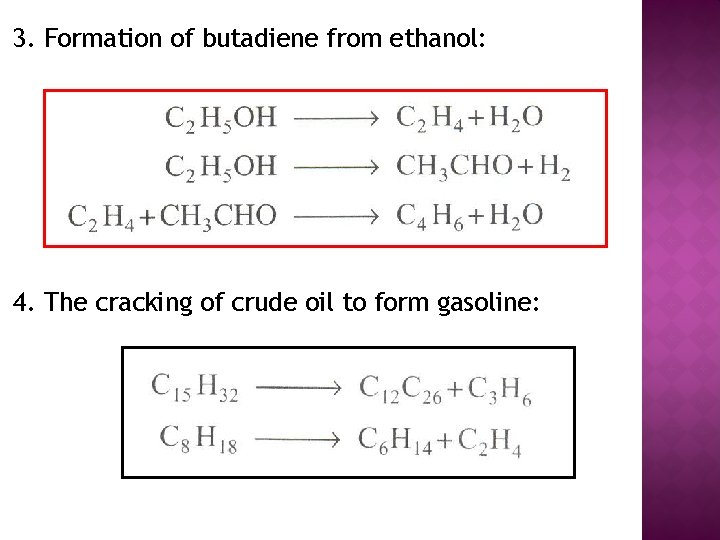
3. Formation of butadiene from ethanol: 4. The cracking of crude oil to form gasoline:
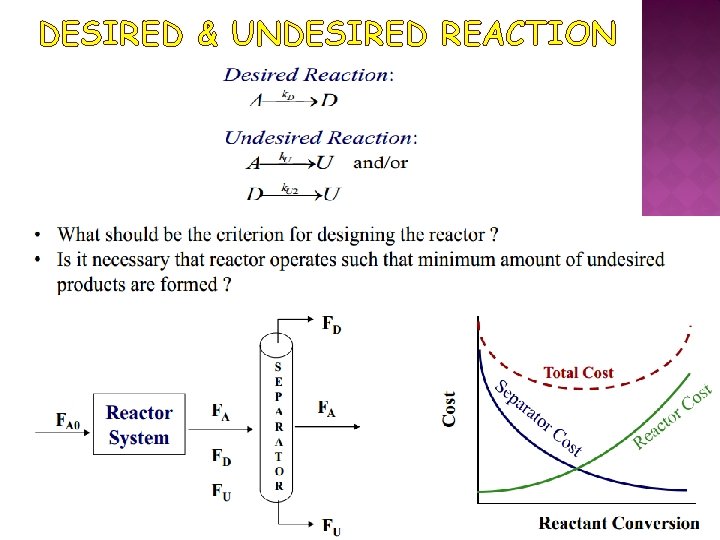
DESIRED & UNDESIRED REACTION
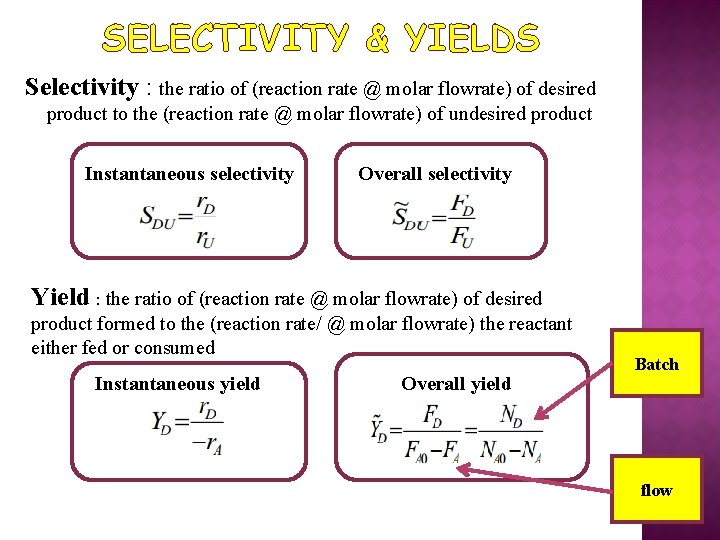
SELECTIVITY & YIELDS Selectivity : the ratio of (reaction rate @ molar flowrate) of desired product to the (reaction rate @ molar flowrate) of undesired product Instantaneous selectivity Overall selectivity Yield : the ratio of (reaction rate @ molar flowrate) of desired product formed to the (reaction rate/ @ molar flowrate) the reactant either fed or consumed Instantaneous yield Overall yield Batch flow
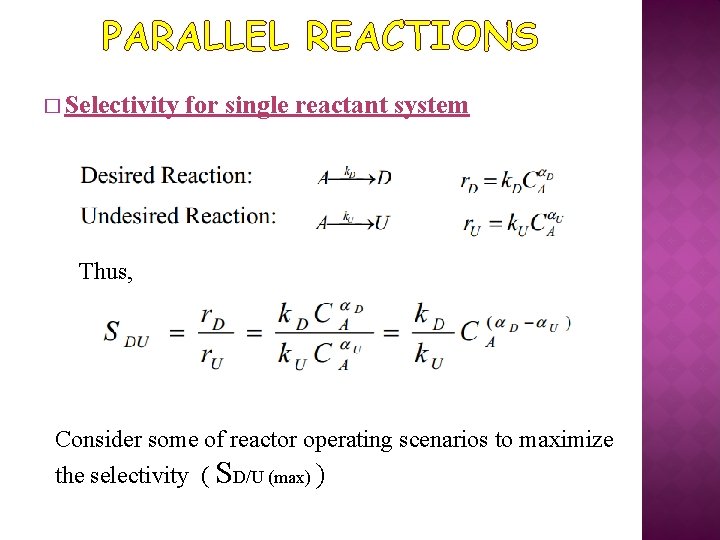
PARALLEL REACTIONS � Selectivity for single reactant system Thus, Consider some of reactor operating scenarios to maximize the selectivity ( SD/U (max) )
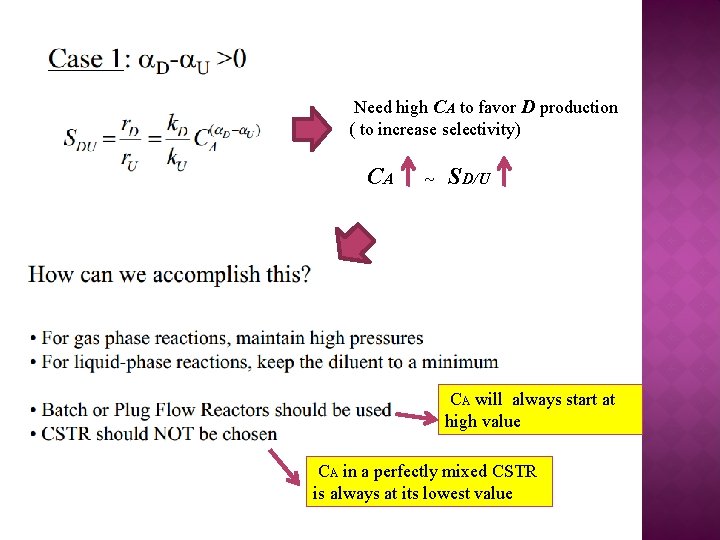
Need high CA to favor D production ( to increase selectivity) CA ~ SD/U CA will always start at high value CA in a perfectly mixed CSTR is always at its lowest value
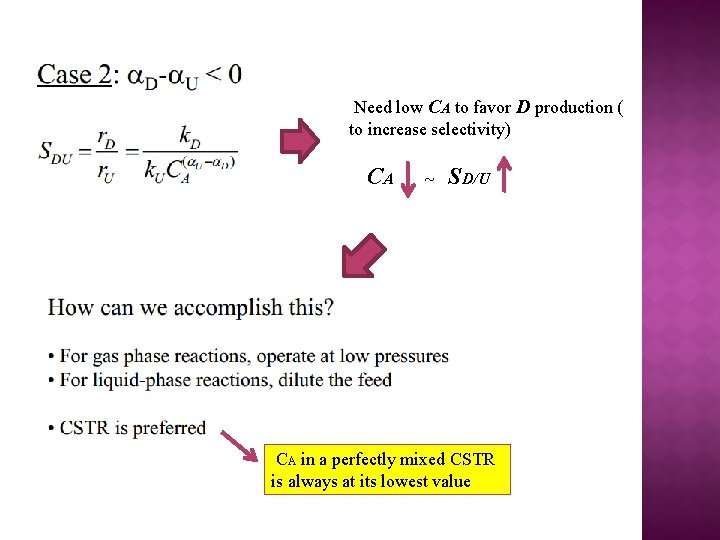
Need low CA to favor D production ( to increase selectivity) CA ~ SD/U CA in a perfectly mixed CSTR is always at its lowest value
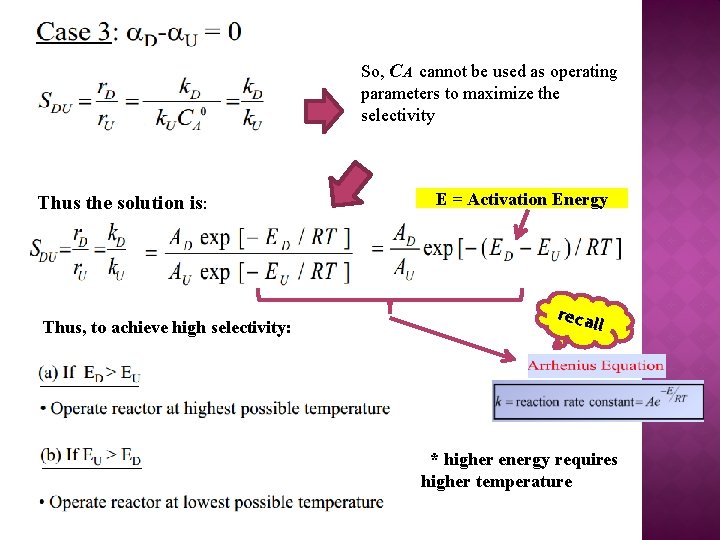
So, CA cannot be used as operating parameters to maximize the selectivity Thus the solution is: Thus, to achieve high selectivity: E = Activation Energy reca l l * higher energy requires higher temperature
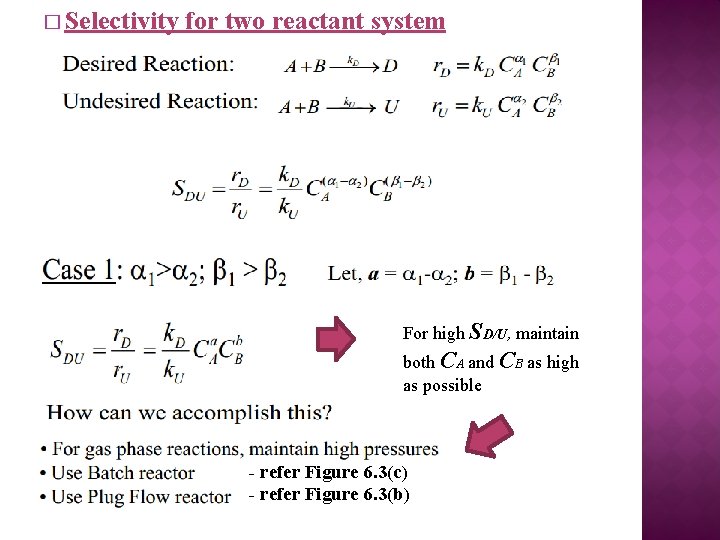
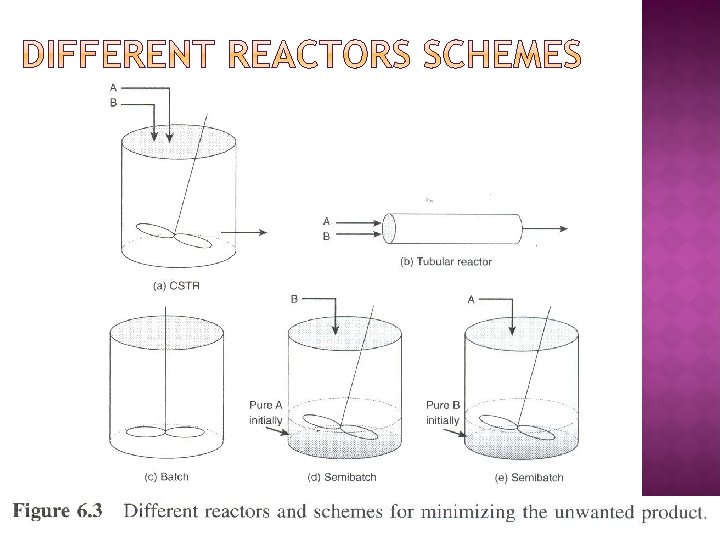
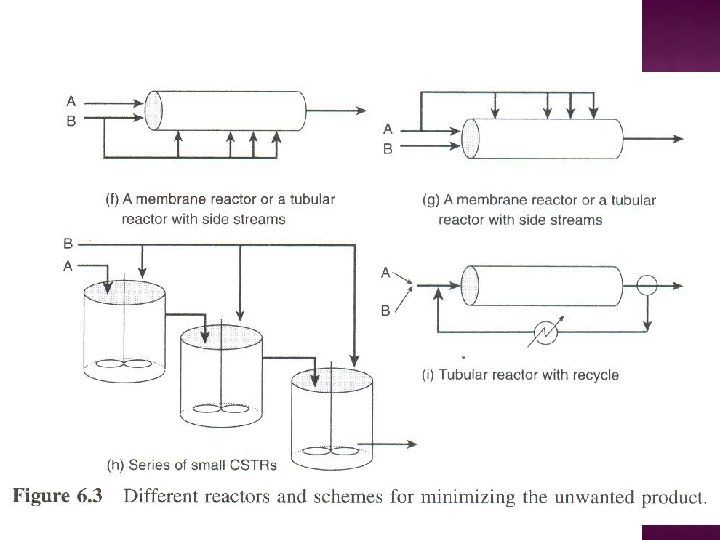
� Selectivity for two reactant system For high SD/U, maintain both CA and CB as high as possible - refer Figure 6. 3(c) - refer Figure 6. 3(b)
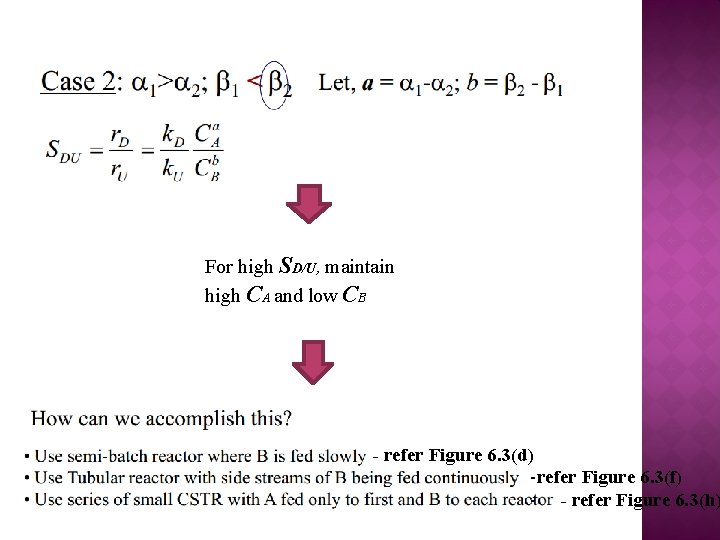
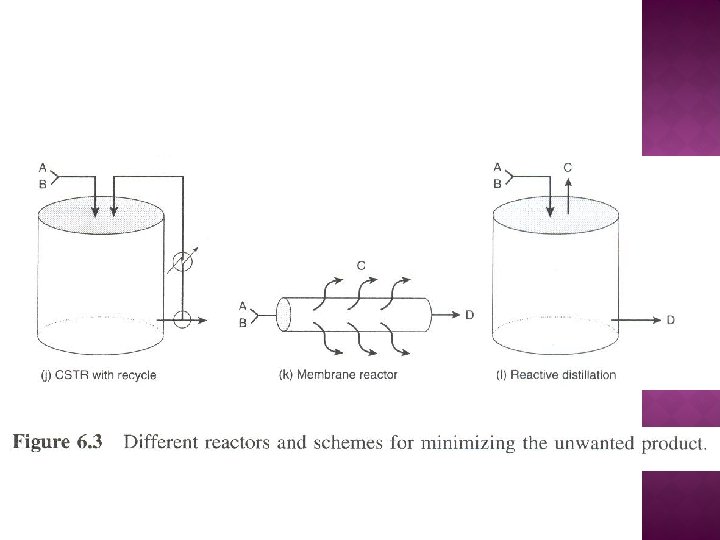
For high SD/U, maintain high CA and low CB - refer Figure 6. 3(d) -refer Figure 6. 3(f) - - refer Figure 6. 3(h)
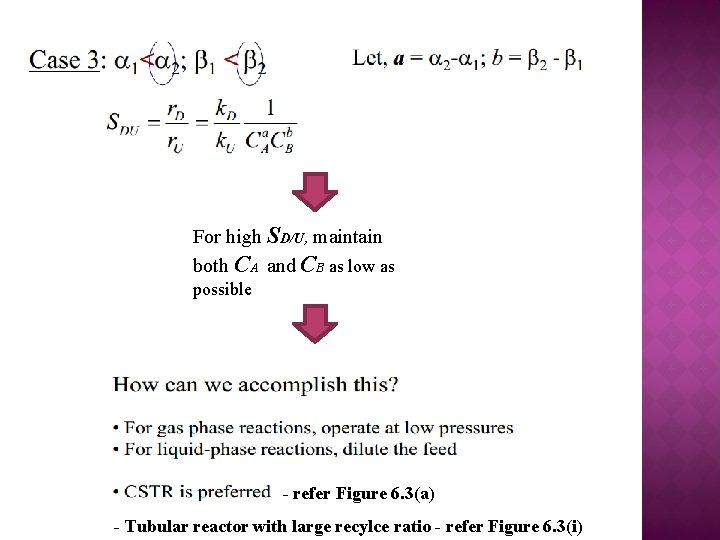
For high SD/U, maintain both CA and CB as low as possible - refer Figure 6. 3(a) - Tubular reactor with large recylce ratio - refer Figure 6. 3(i)
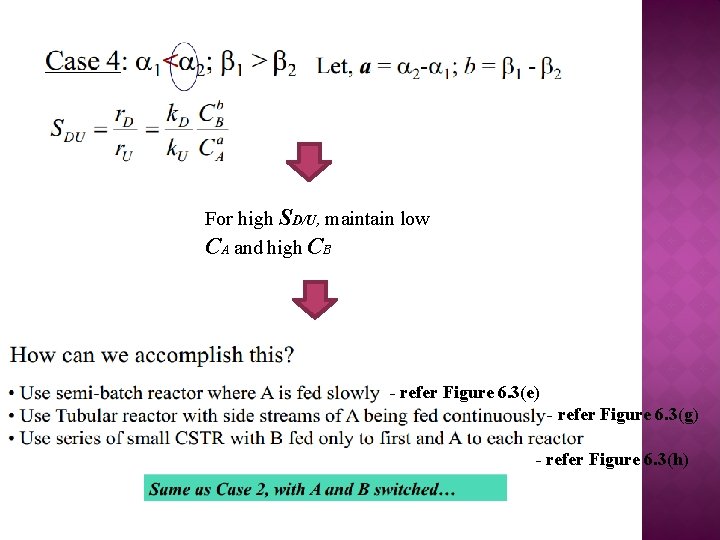
For high SD/U, maintain low CA and high CB - refer Figure 6. 3(e) - refer Figure 6. 3(g) - refer Figure 6. 3(h)

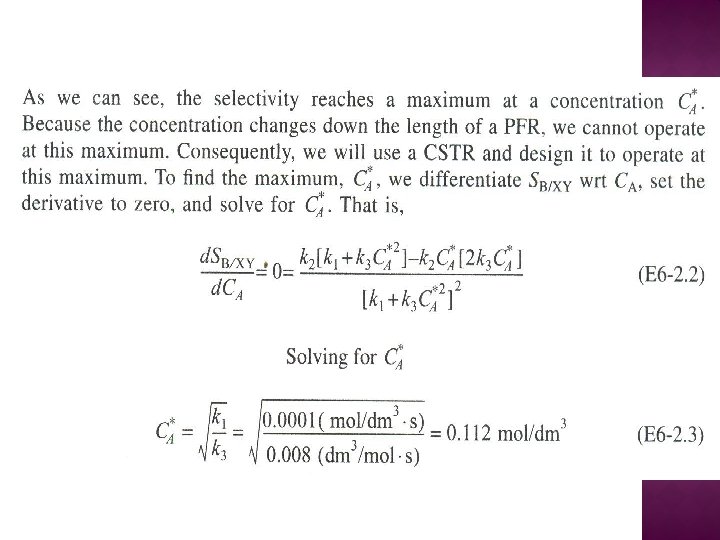
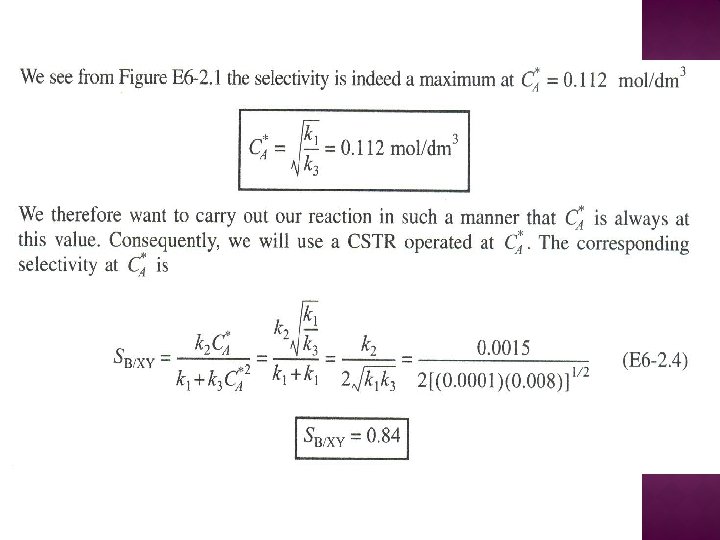
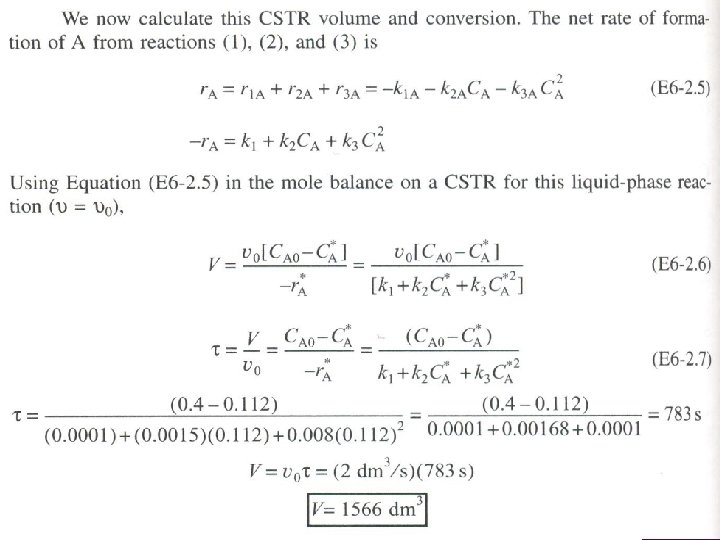
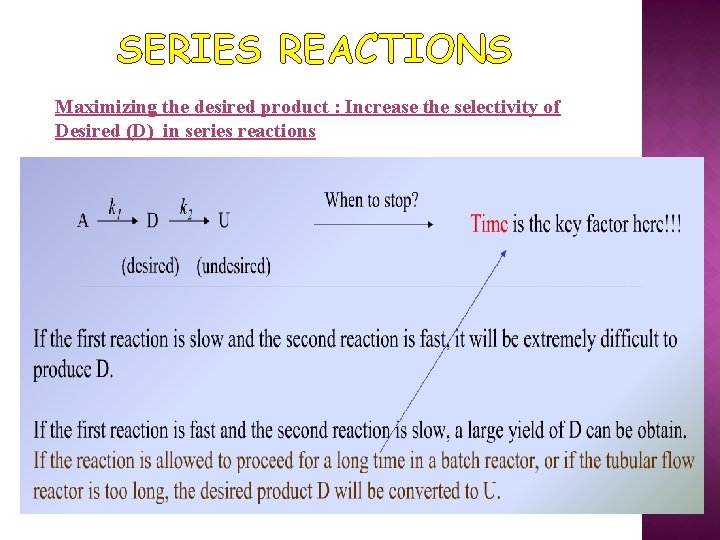
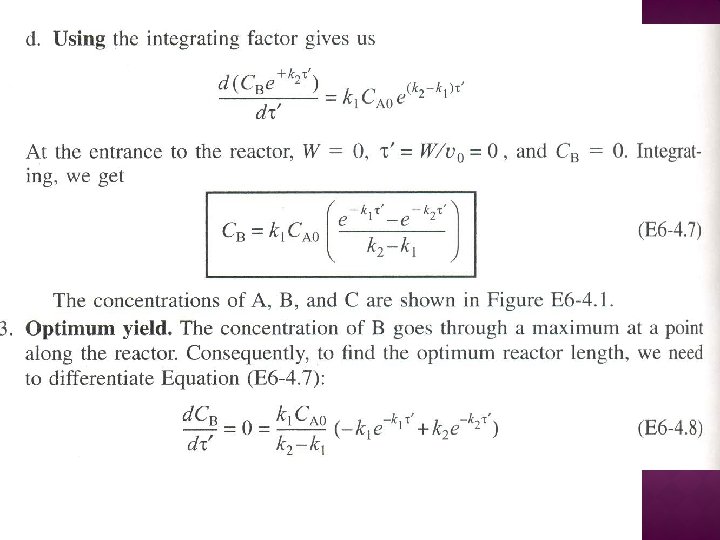
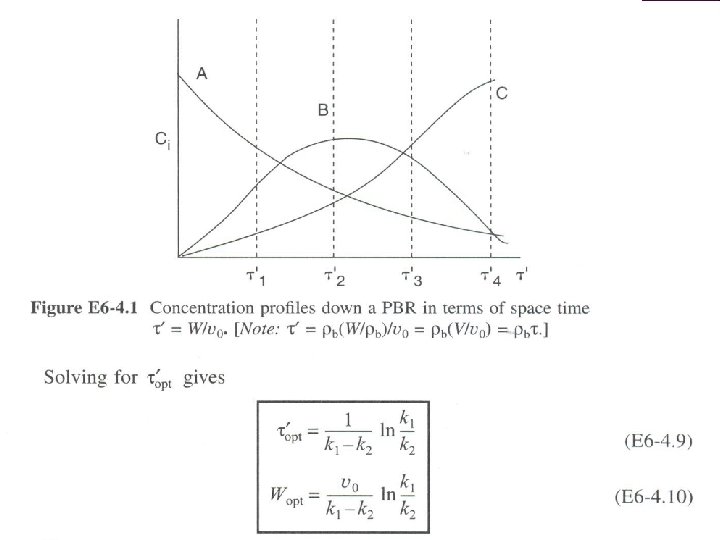
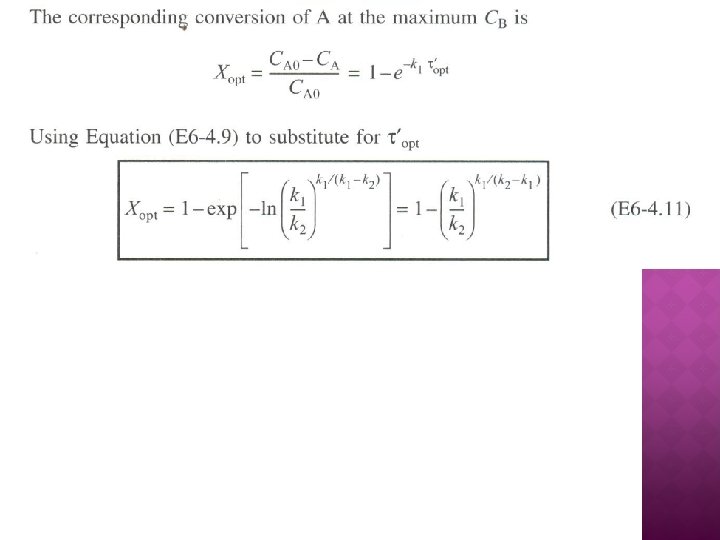
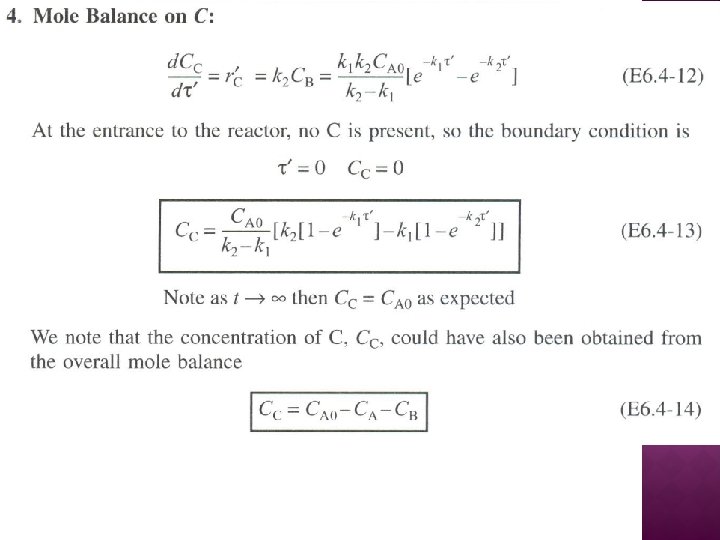
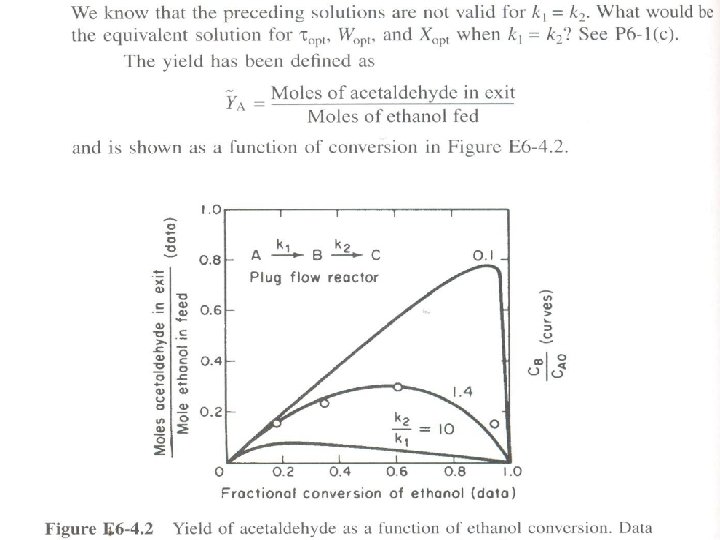
SERIES REACTIONS Maximizing the desired product : Increase the selectivity of Desired (D) in series reactions
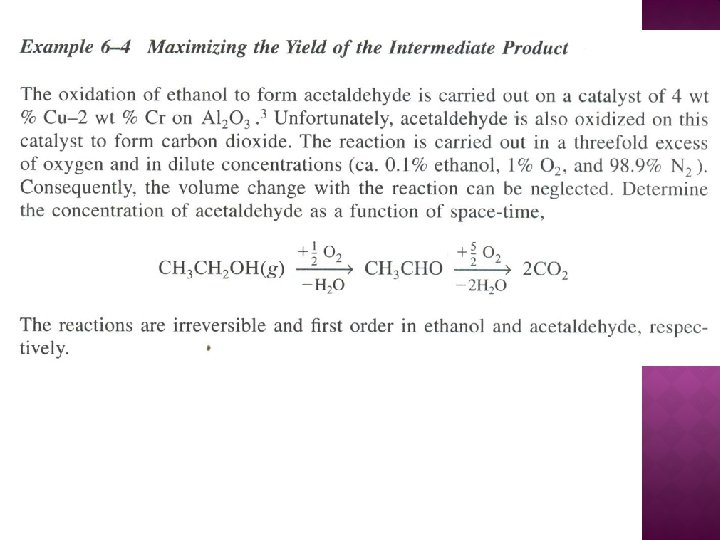
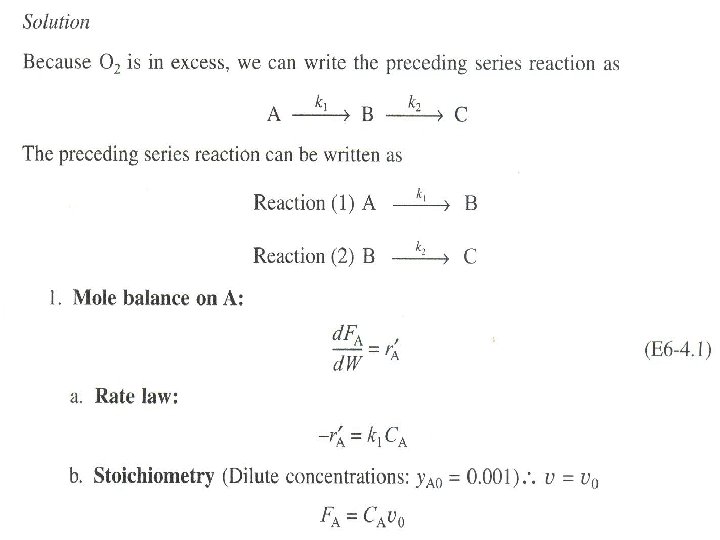
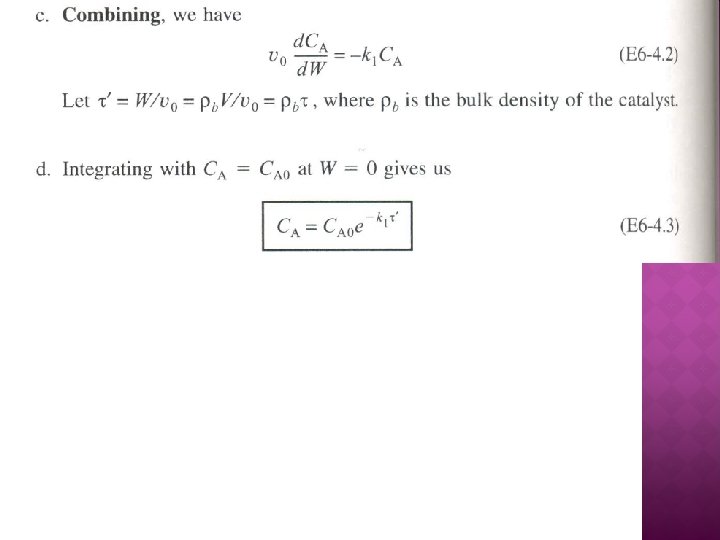
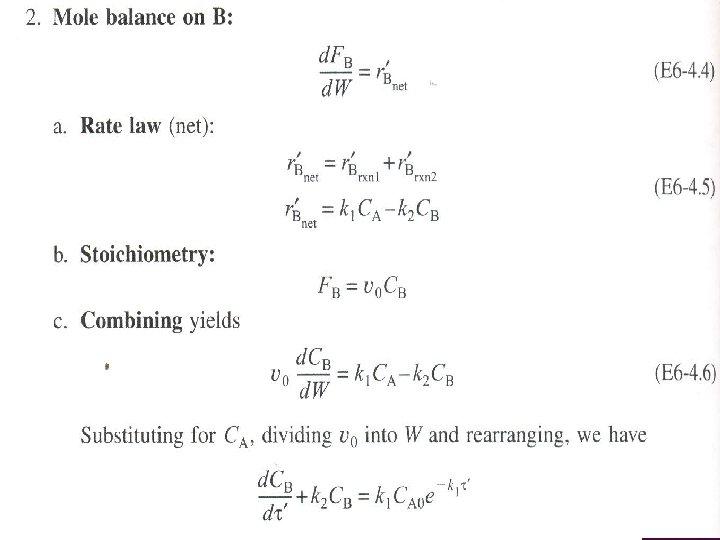
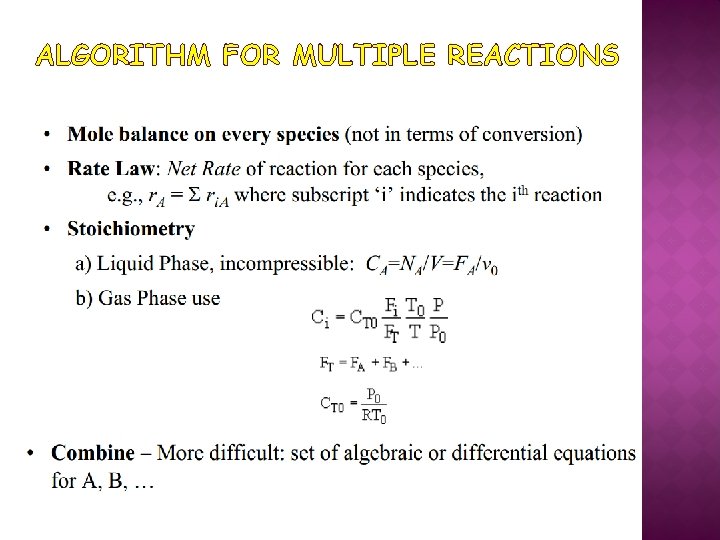
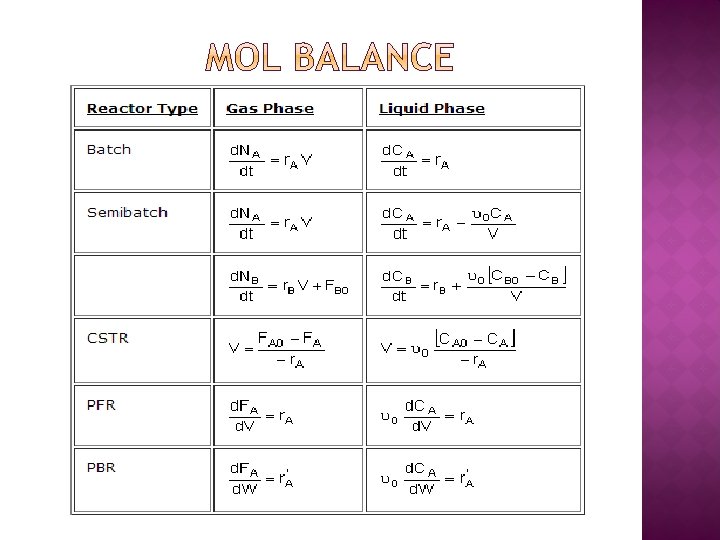
ALGORITHM FOR MULTIPLE REACTIONS

ALGORITHM FOR COMPLEX REACTIONS
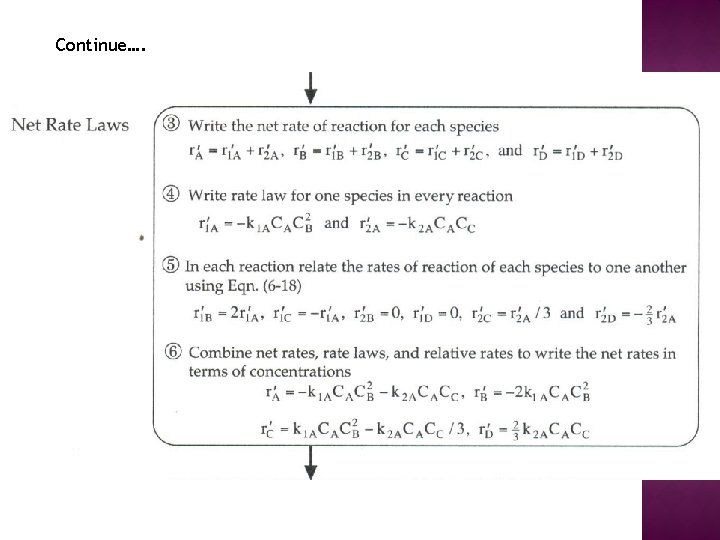
Continue….
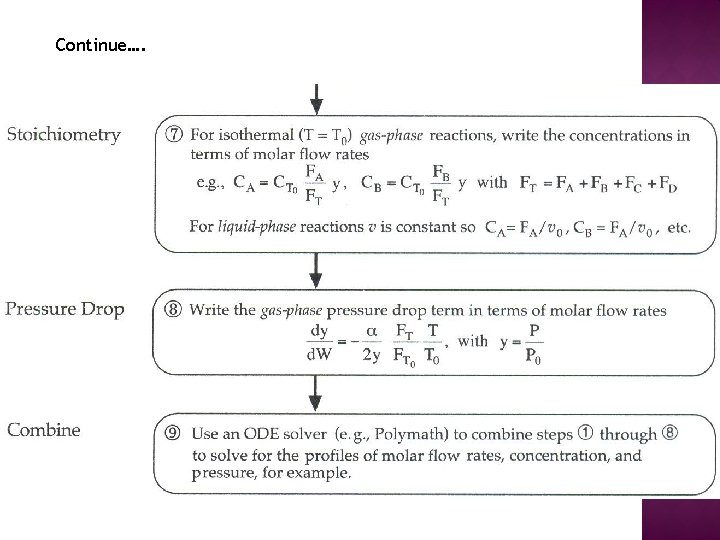
Continue….
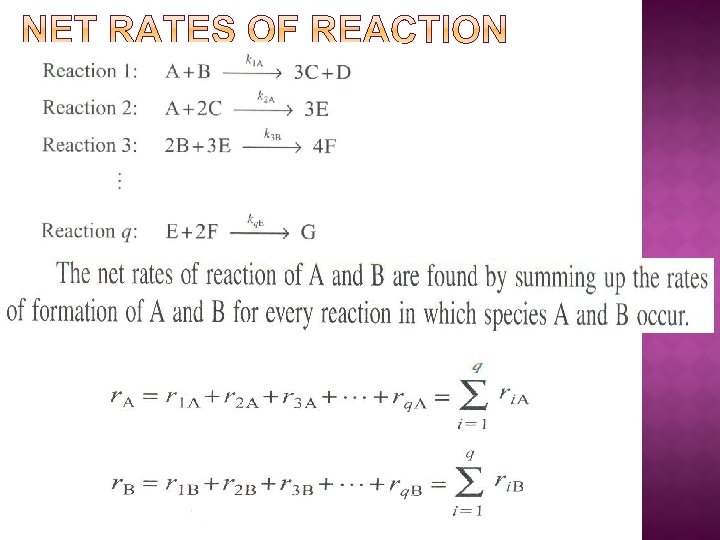
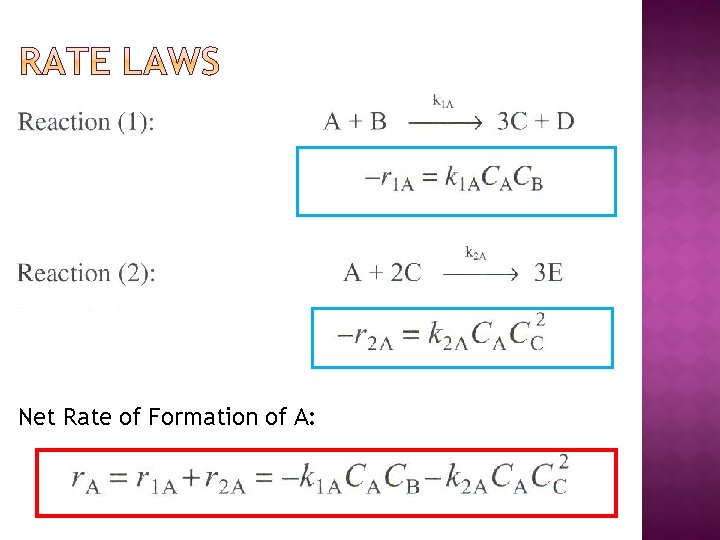
Net Rate of Formation of A:
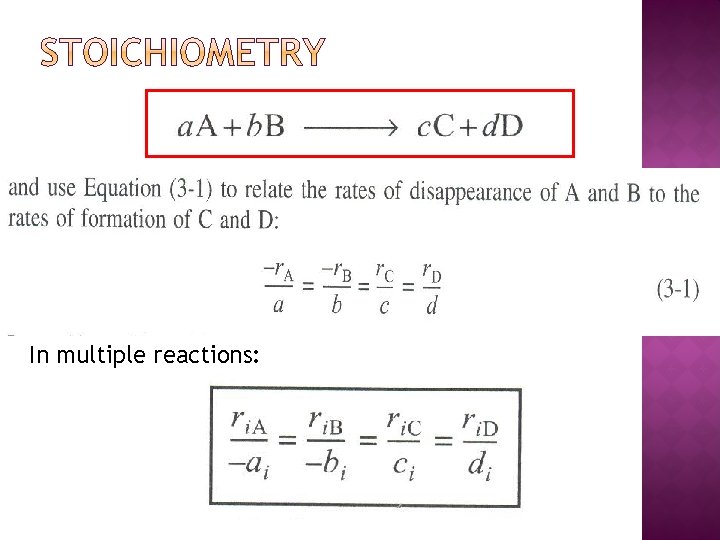
In multiple reactions:
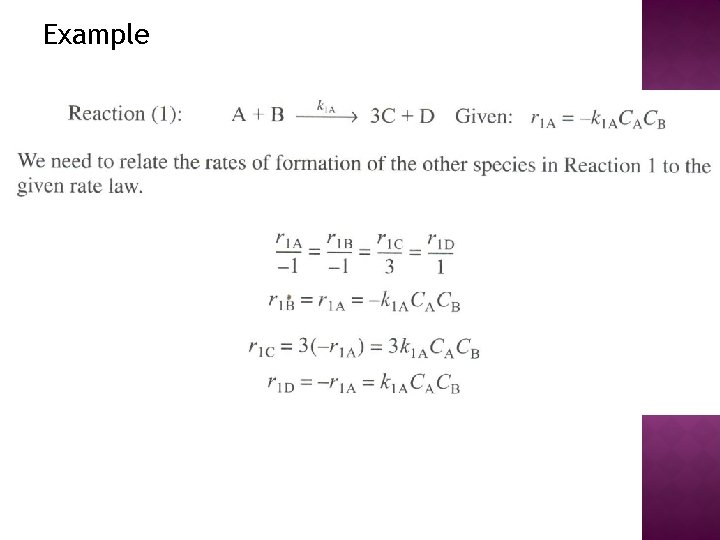
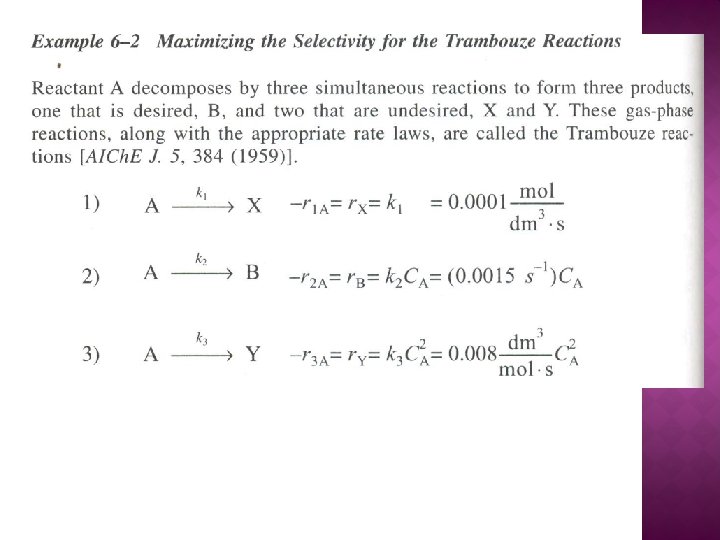
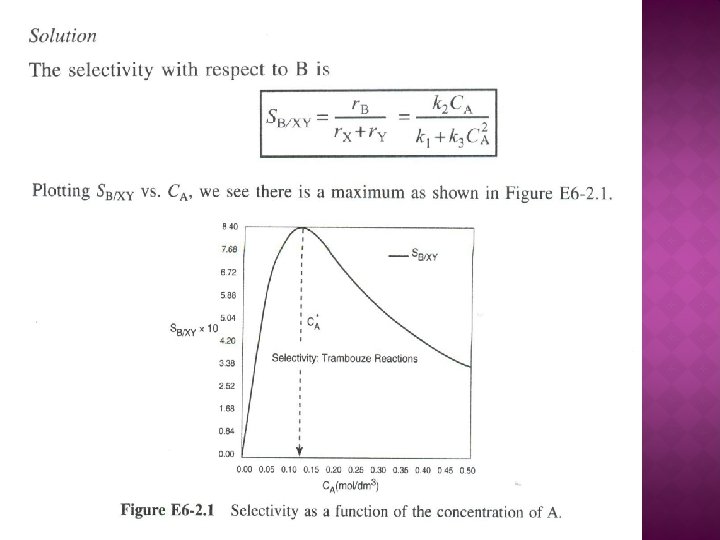
Example
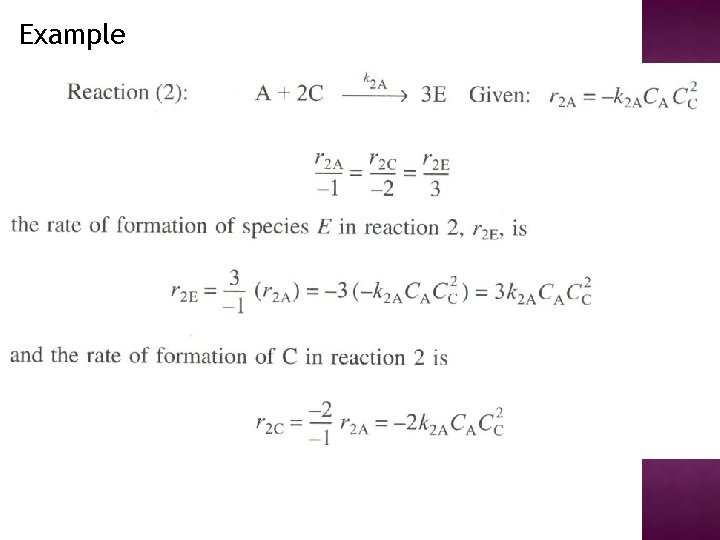
Example
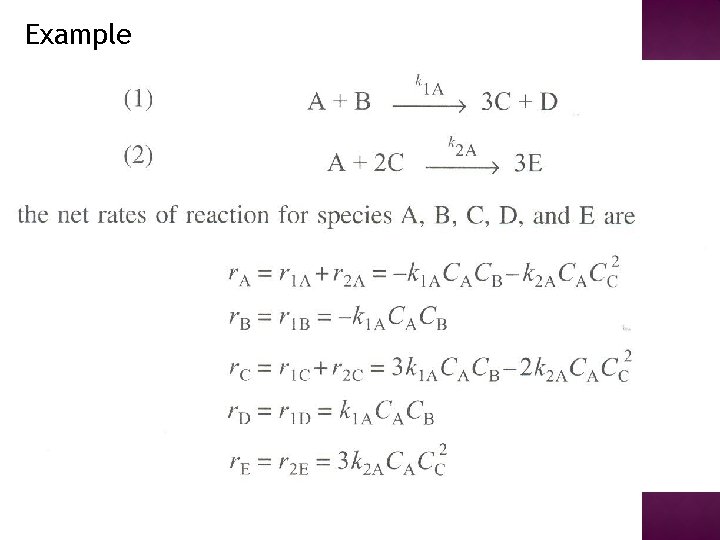
Example
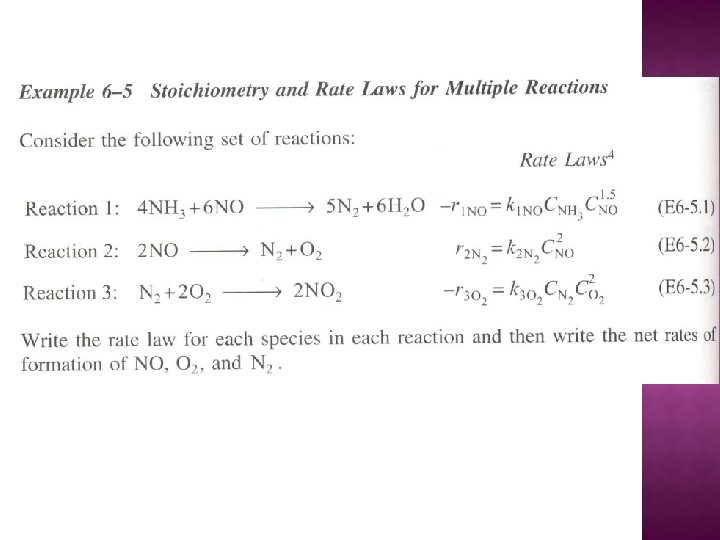
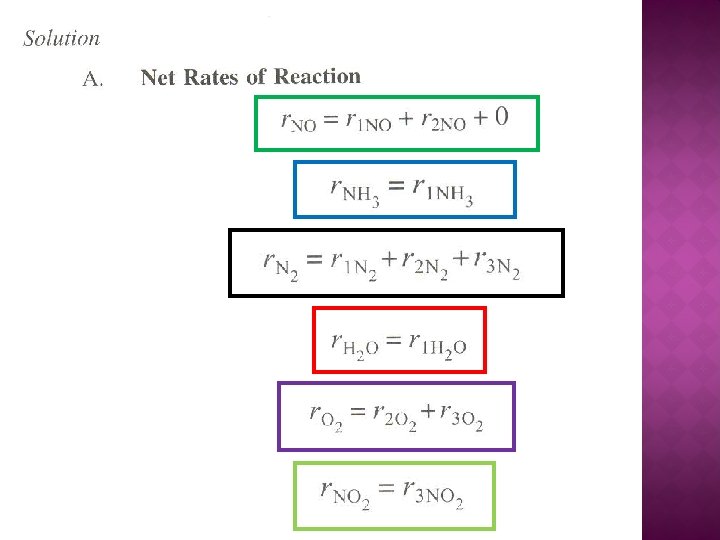
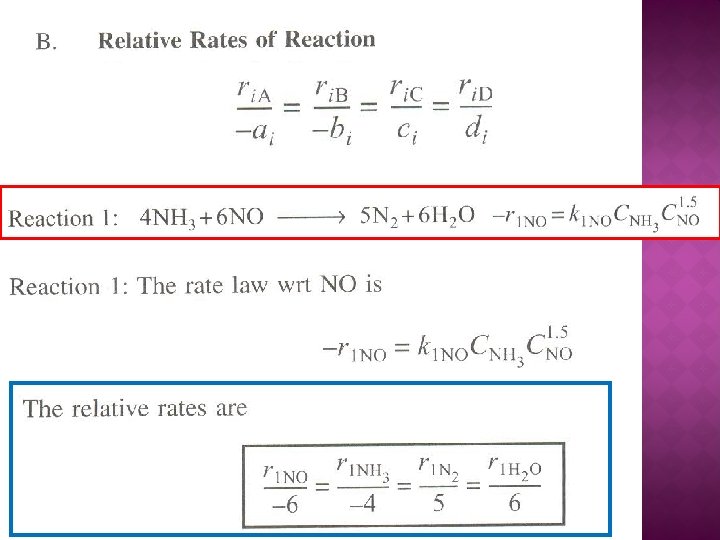
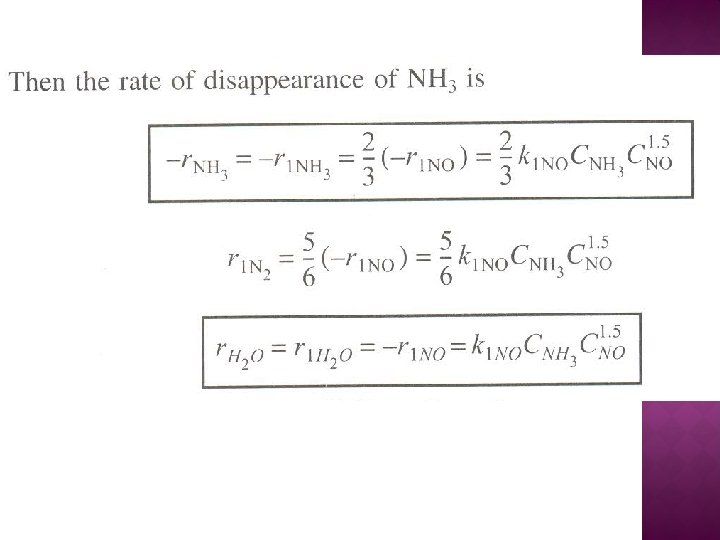
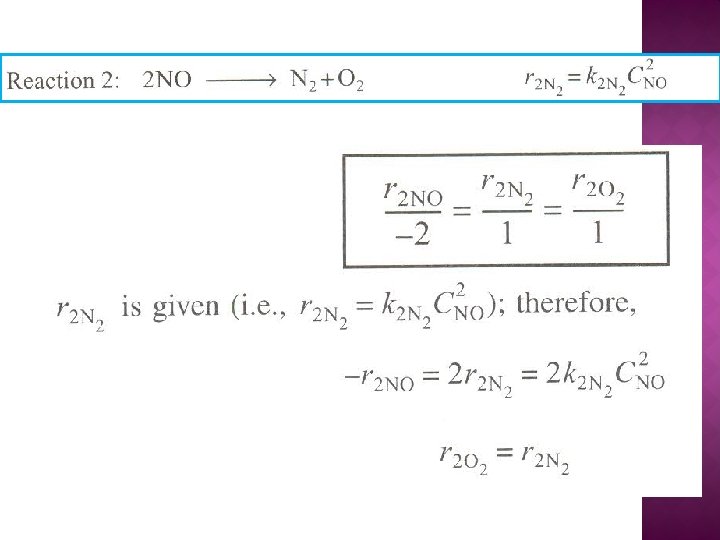
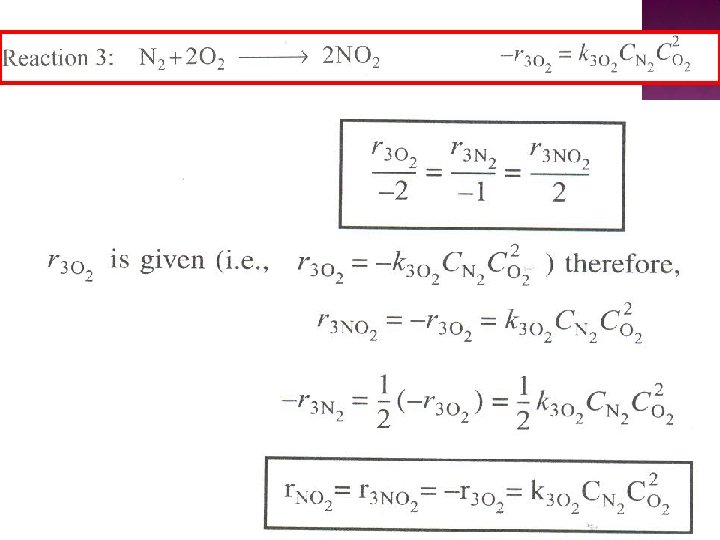
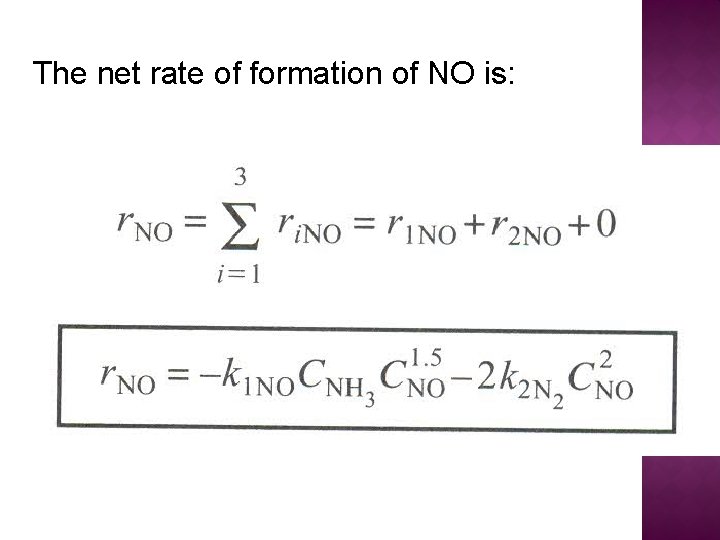
The net rate of formation of NO is:
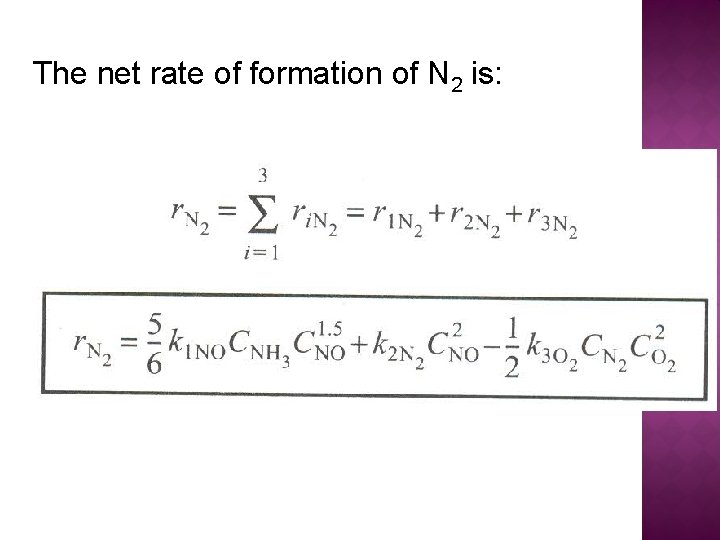
The net rate of formation of N 2 is:
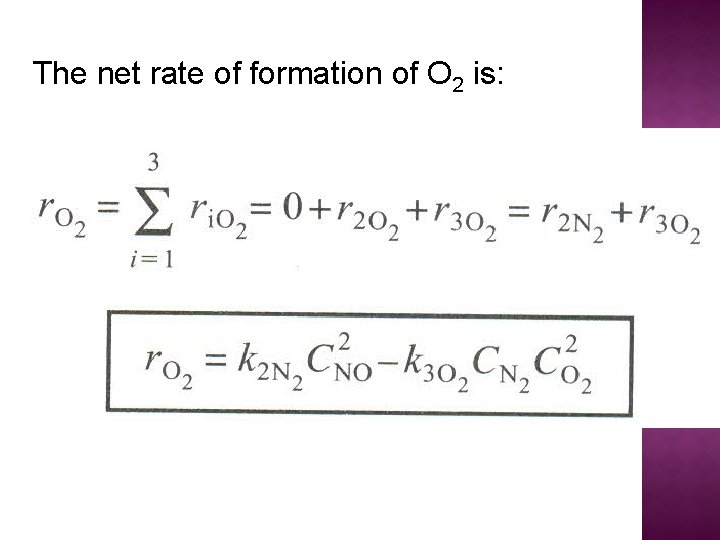
The net rate of formation of O 2 is:

- Slides: 56