MULTIPLE REACTIONS By Noor Amirah Abdul Halim TYPE
MULTIPLE REACTIONS By Noor Amirah Abdul Halim
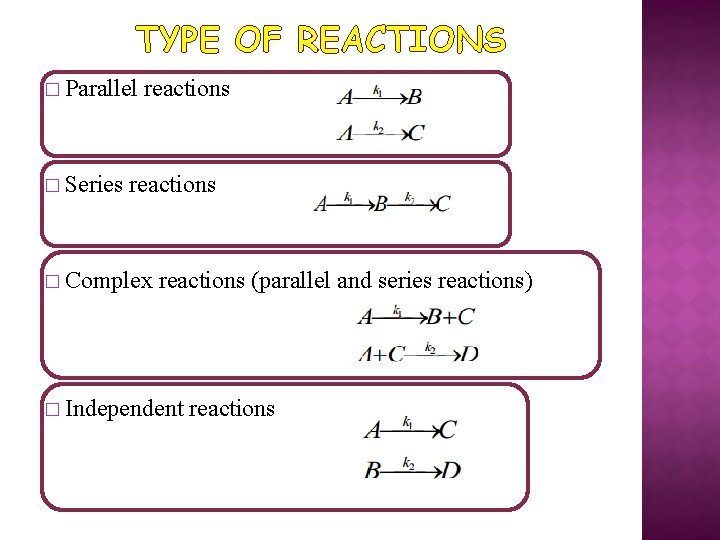
TYPE OF REACTIONS � Parallel � Series reactions � Complex reactions (parallel and series reactions) � Independent reactions
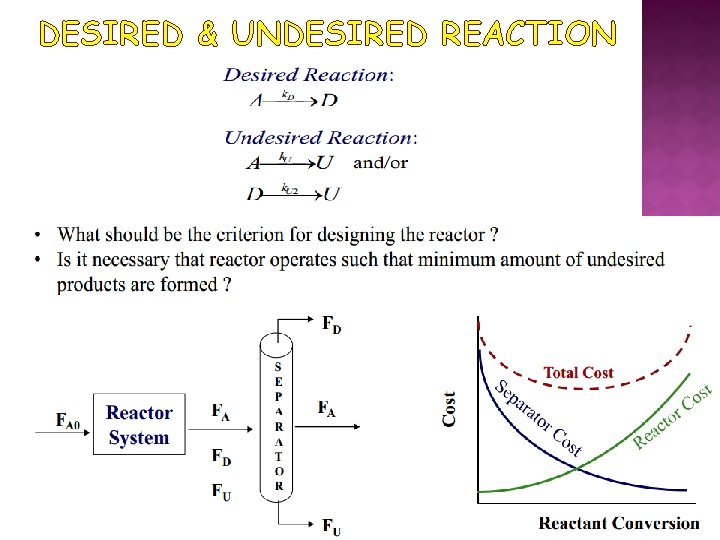
DESIRED & UNDESIRED REACTION
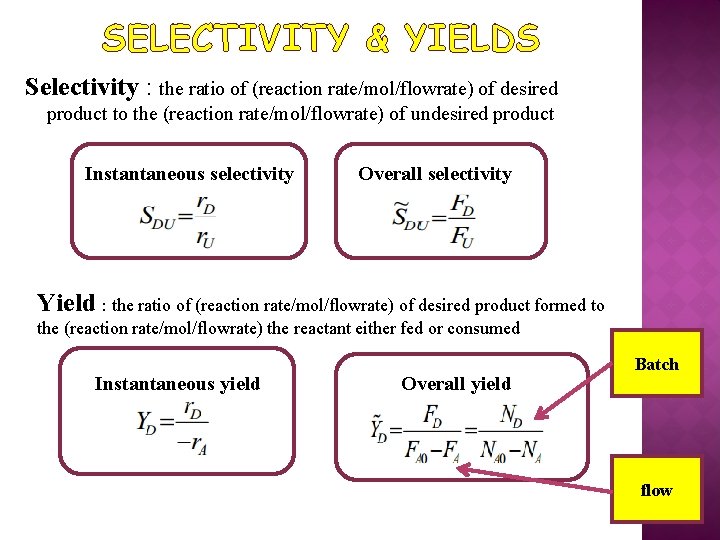
SELECTIVITY & YIELDS Selectivity : the ratio of (reaction rate/mol/flowrate) of desired product to the (reaction rate/mol/flowrate) of undesired product Instantaneous selectivity Overall selectivity Yield : the ratio of (reaction rate/mol/flowrate) of desired product formed to the (reaction rate/mol/flowrate) the reactant either fed or consumed Instantaneous yield Overall yield Batch flow
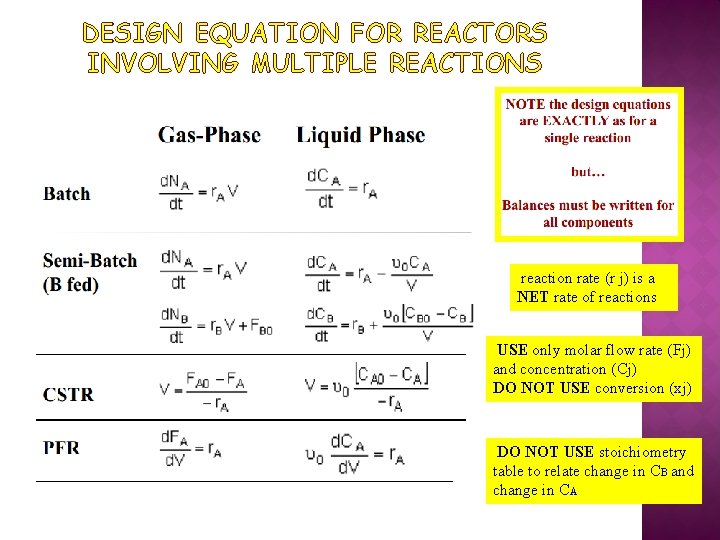
DESIGN EQUATION FOR REACTORS INVOLVING MULTIPLE REACTIONS reaction rate (r j) is a NET rate of reactions USE only molar flow rate (Fj) and concentration (Cj) DO NOT USE conversion (xj) DO NOT USE stoichiometry table to relate change in CB and change in CA
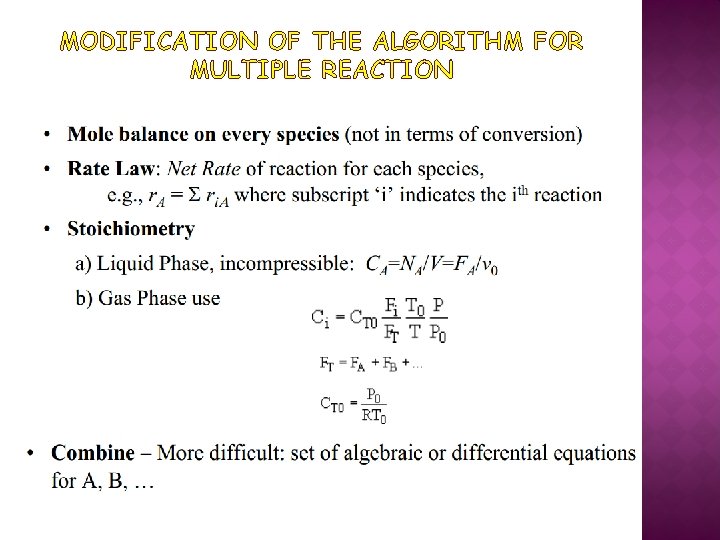
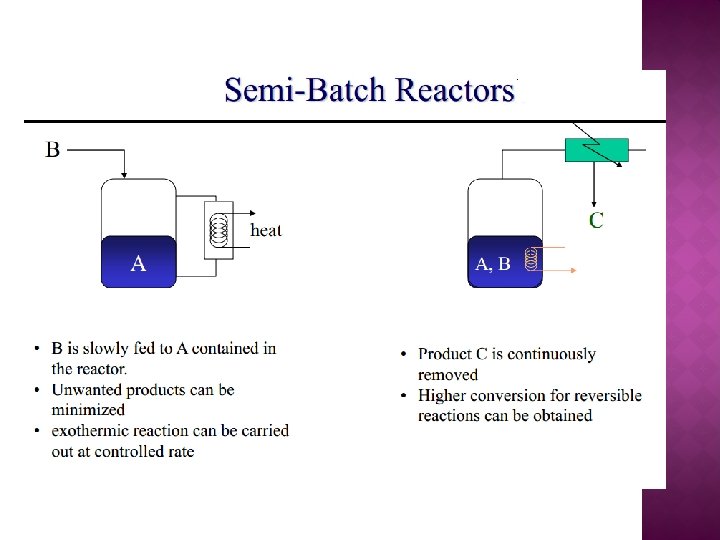
MODIFICATION OF THE ALGORITHM FOR MULTIPLE REACTION
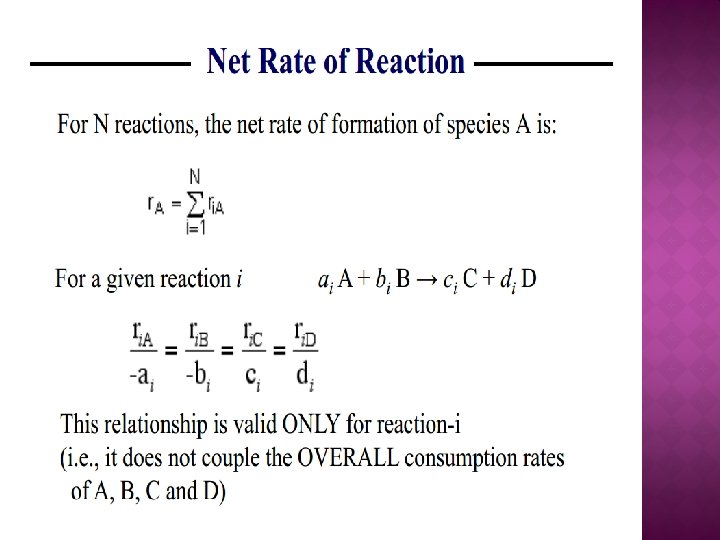
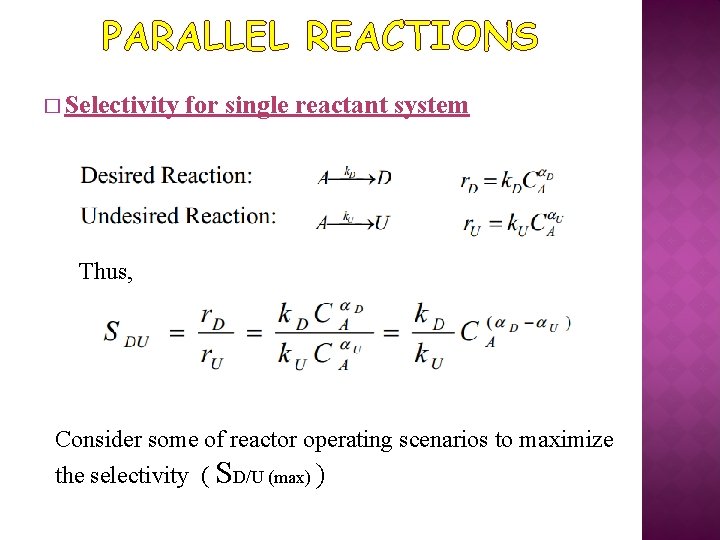
PARALLEL REACTIONS � Selectivity for single reactant system Thus, Consider some of reactor operating scenarios to maximize the selectivity ( SD/U (max) )
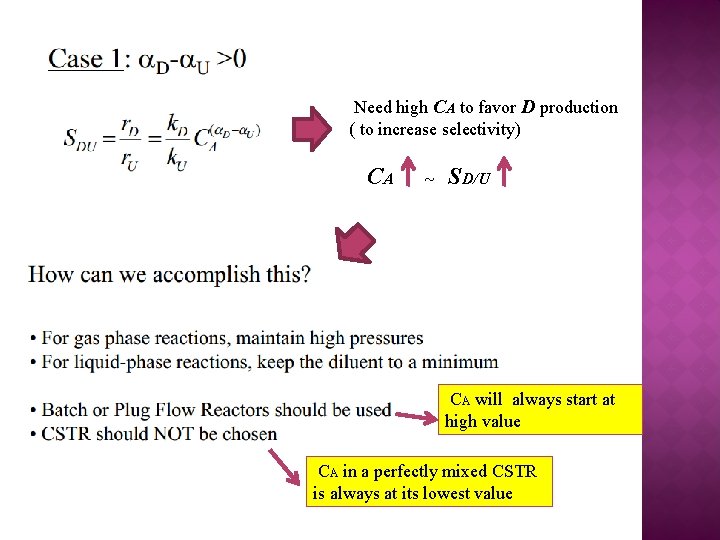
Need high CA to favor D production ( to increase selectivity) CA ~ SD/U CA will always start at high value CA in a perfectly mixed CSTR is always at its lowest value
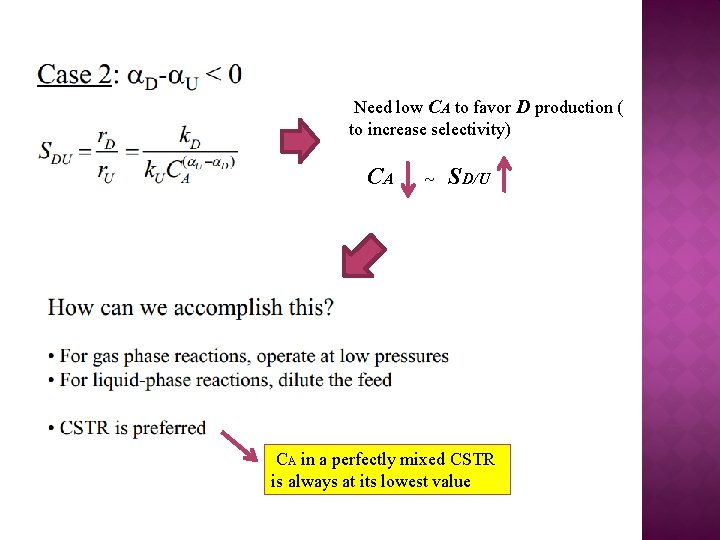
Need low CA to favor D production ( to increase selectivity) CA ~ SD/U CA in a perfectly mixed CSTR is always at its lowest value
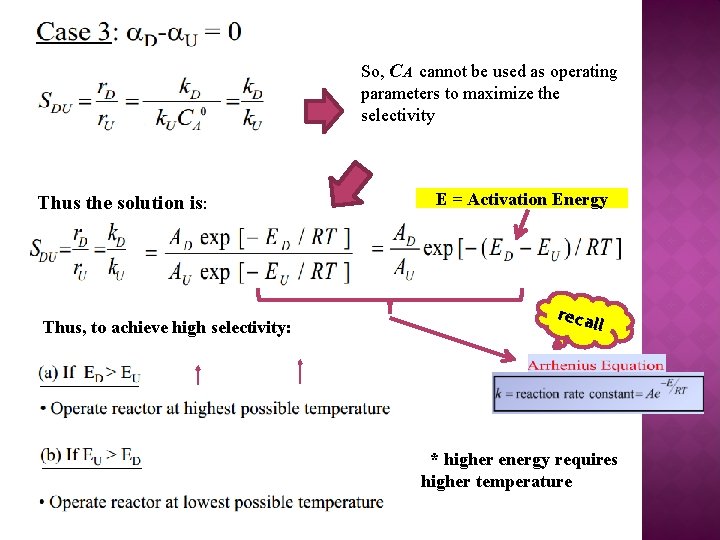
So, CA cannot be used as operating parameters to maximize the selectivity Thus the solution is: Thus, to achieve high selectivity: E = Activation Energy reca l l * higher energy requires higher temperature
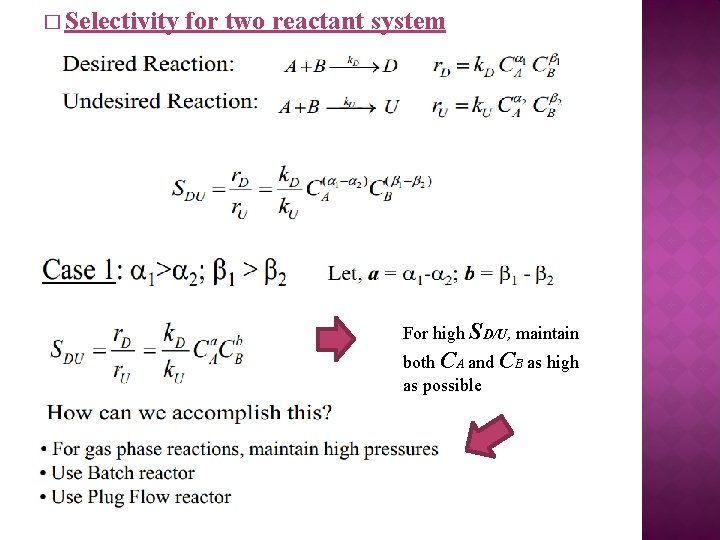
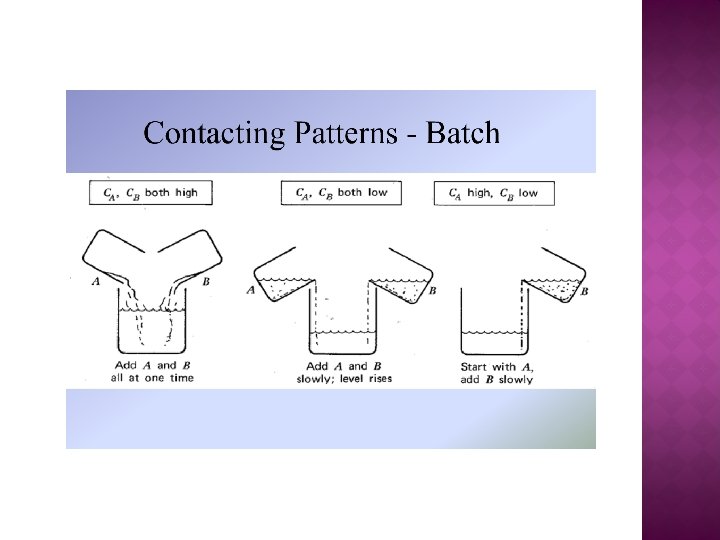
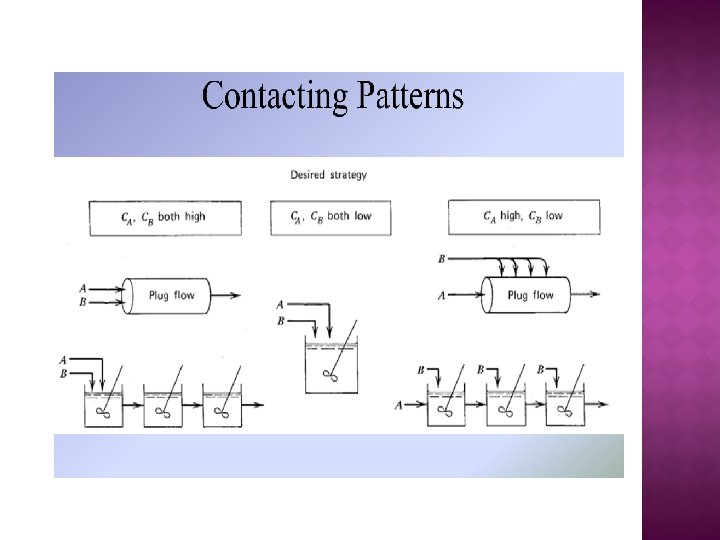
� Selectivity for two reactant system For high SD/U, maintain both CA and CB as high as possible
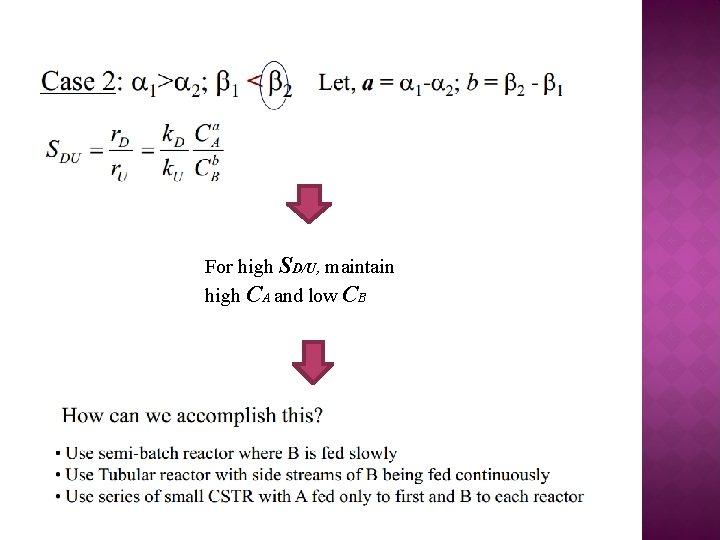
For high SD/U, maintain high CA and low CB
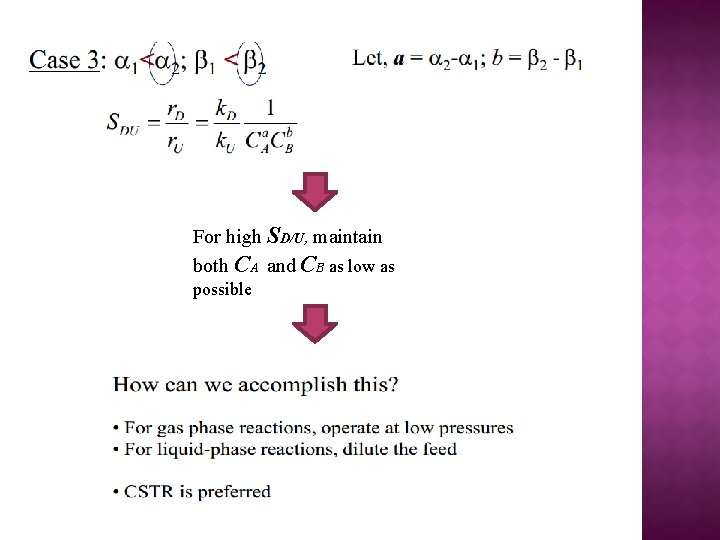
For high SD/U, maintain both CA and CB as low as possible
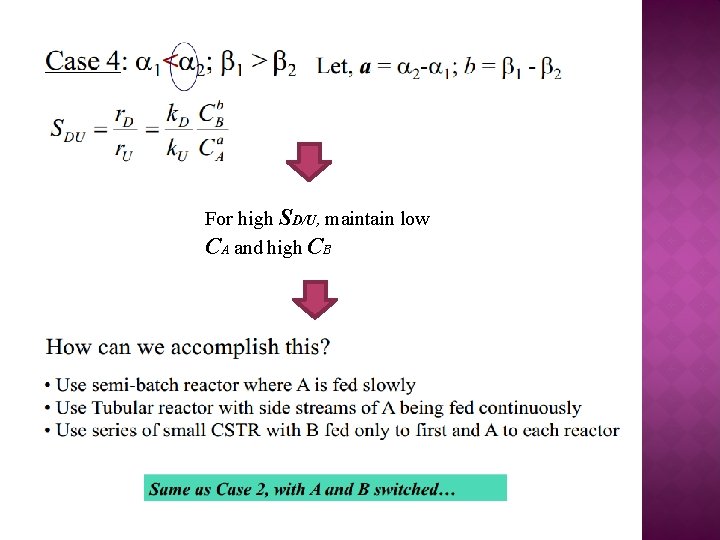
For high SD/U, maintain low CA and high CB
- Slides: 19