Multiple Oxidation States Truman Chemistry Dept Oxidation States



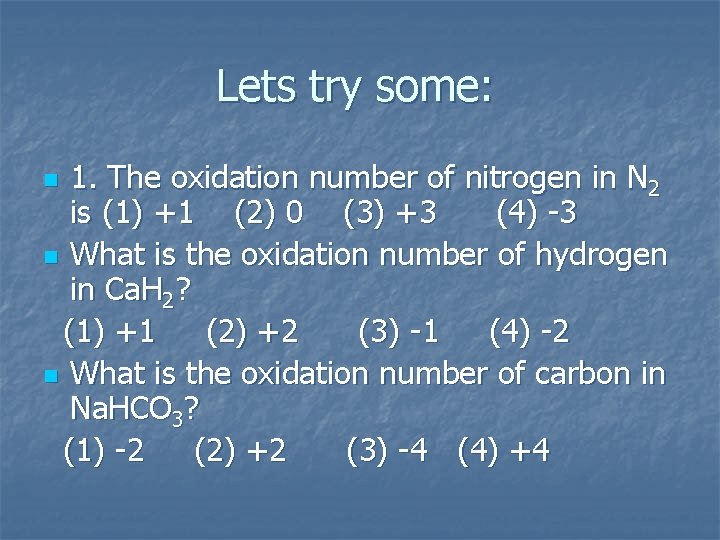

- Slides: 8

Multiple Oxidation States Truman Chemistry Dept.

Oxidation States can change during a reaction n Example: Oxidation state of oxygen n CH 4 + 2 O 2 CO 2 + 2 H 2 O In O 2 the oxidation state of O is zero n In CO 2 and H 2 O the oxidation state of O is -2 n So the main question here is how can we predict how the oxidation state will change? n There are rules to predict this…. n

Rules n n All elements in a diatomic molecule have an oxidation state of zero (they are neutral) ie. Cl 2 If an element is monatomic (by itself) the charge is only as specified (ie. Cl-). Free elements have a charge of zero (ie. Fe) n All neutral compounds must have a net (total) oxidation charge of zero and the element with the highest electronegativity (furthest right on table) is the negative element.

Rules continued… n n n Hydrogen always has a +1 charge except in hydrides where it is negative one (ie. Li. H or Ca. H 2) Oxygen always has a -2 charge except in peroxides (H 2 O 2) where it is -1 Group 1 elements are normally +1 Group 2 elements are normally +2 Group 17 elements are often -1

Polyatomic ion rules… n Polyatomic ions have a net charge that is equal to the sum of all the charges.
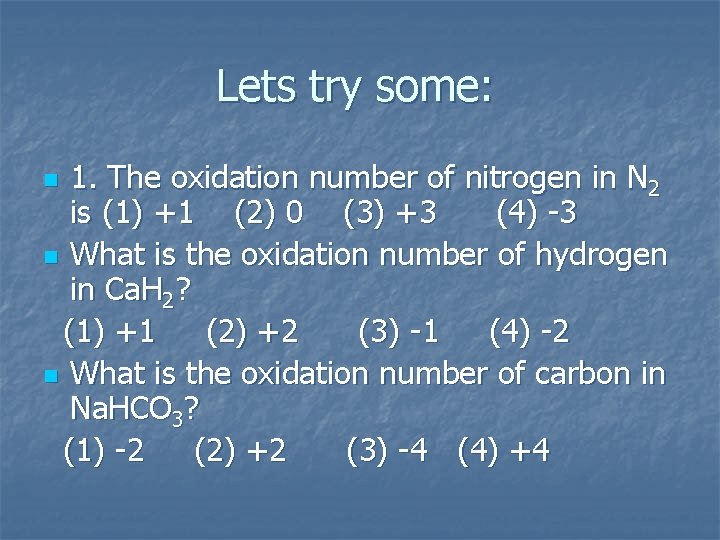
Lets try some: 1. The oxidation number of nitrogen in N 2 is (1) +1 (2) 0 (3) +3 (4) -3 n What is the oxidation number of hydrogen in Ca. H 2? (1) +1 (2) +2 (3) -1 (4) -2 n What is the oxidation number of carbon in Na. HCO 3? (1) -2 (2) +2 (3) -4 (4) +4 n
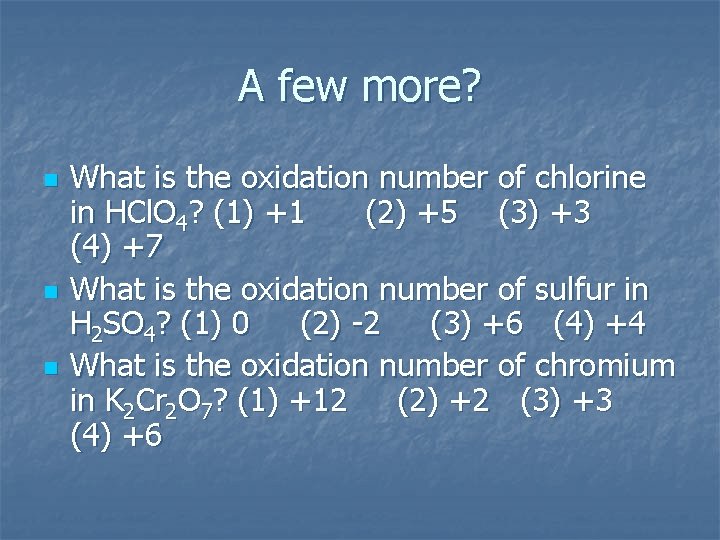
A few more? n n n What is the oxidation number of chlorine in HCl. O 4? (1) +1 (2) +5 (3) +3 (4) +7 What is the oxidation number of sulfur in H 2 SO 4? (1) 0 (2) -2 (3) +6 (4) +4 What is the oxidation number of chromium in K 2 Cr 2 O 7? (1) +12 (2) +2 (3) +3 (4) +6

Lets look at some reactions: n Al + O 2 Al 2 O 3 n n n What happened to the oxidation states? Half reactions? S + NO 3 - SO 2 + NO n What happened to the oxidation states?