Multiple Lab Units Representation By Multiple Lab Units
Multiple Lab Units Representation By Multiple Lab Units Representation Team

Problem Statement • The FDA Position on Use of SI Units for Lab Tests (https: //www. fda. gov/downloads/For. Industry/Data. Standards/Study Data. Standards/UCM 587505. pdf) states that SI units are the preferred units for submissions. • It also states that an FDA review division could request a sponsor to submit (some) lab values in US conventional units. • The CDISC user community would like clarity on how multiple lab units should be submitted to the FDA, if requested. • There a number of options on the following slides.

Options
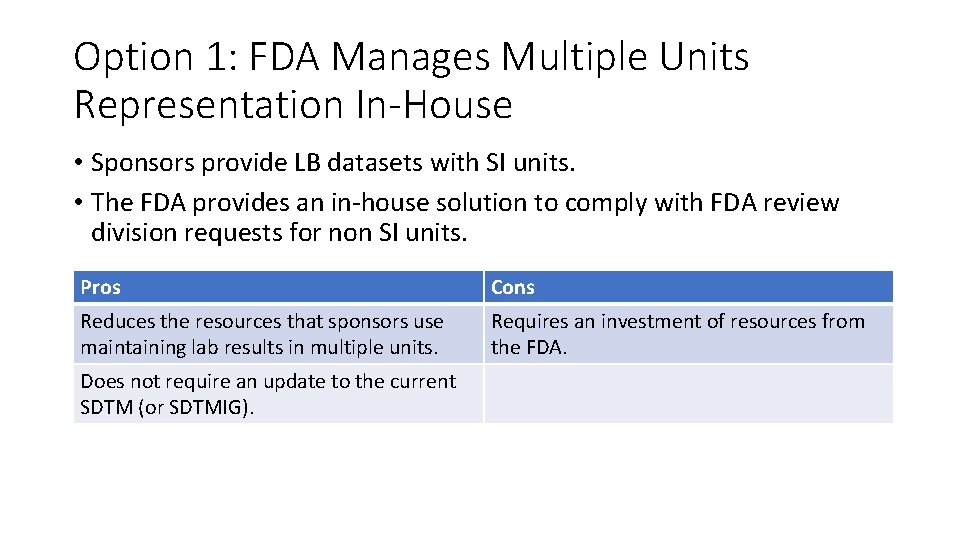
Option 1: FDA Manages Multiple Units Representation In-House • Sponsors provide LB datasets with SI units. • The FDA provides an in-house solution to comply with FDA review division requests for non SI units. Pros Cons Reduces the resources that sponsors use maintaining lab results in multiple units. Requires an investment of resources from the FDA. Does not require an update to the current SDTM (or SDTMIG).
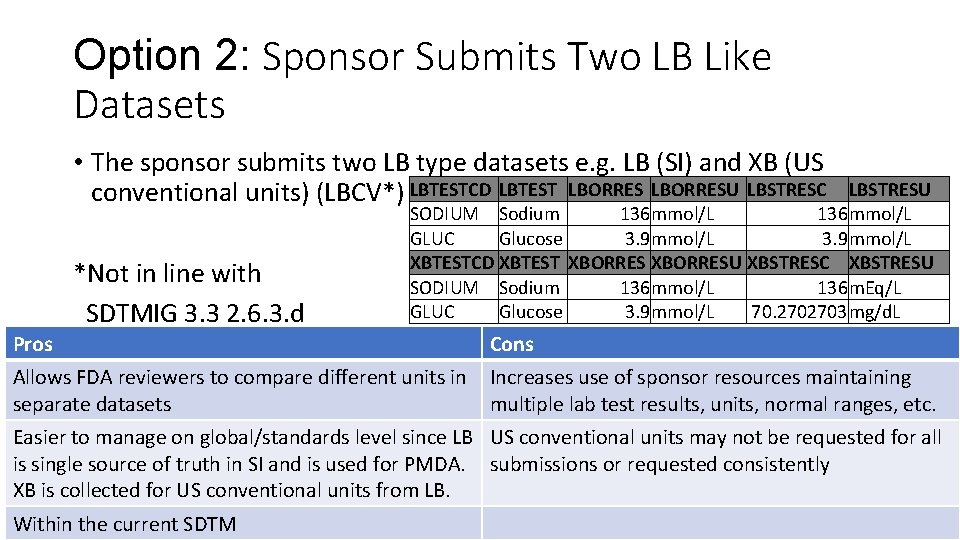
Option 2: Sponsor Submits Two LB Like Datasets • The sponsor submits two LB type datasets e. g. LB (SI) and XB (US conventional units) (LBCV*) LBTESTCD LBTEST LBORRESU LBSTRESC *Not in line with SDTMIG 3. 3 2. 6. 3. d LBSTRESU SODIUM Sodium 136 mmol/L GLUC Glucose 3. 9 mmol/L XBTESTCD XBTEST XBORRESU XBSTRESC XBSTRESU SODIUM Sodium 136 mmol/L 136 m. Eq/L GLUC Glucose 3. 9 mmol/L 70. 2702703 mg/d. L Pros Cons Allows FDA reviewers to compare different units in separate datasets Increases use of sponsor resources maintaining multiple lab test results, units, normal ranges, etc. Easier to manage on global/standards level since LB US conventional units may not be requested for all is single source of truth in SI and is used for PMDA. submissions or requested consistently XB is collected for US conventional units from LB. Within the current SDTM
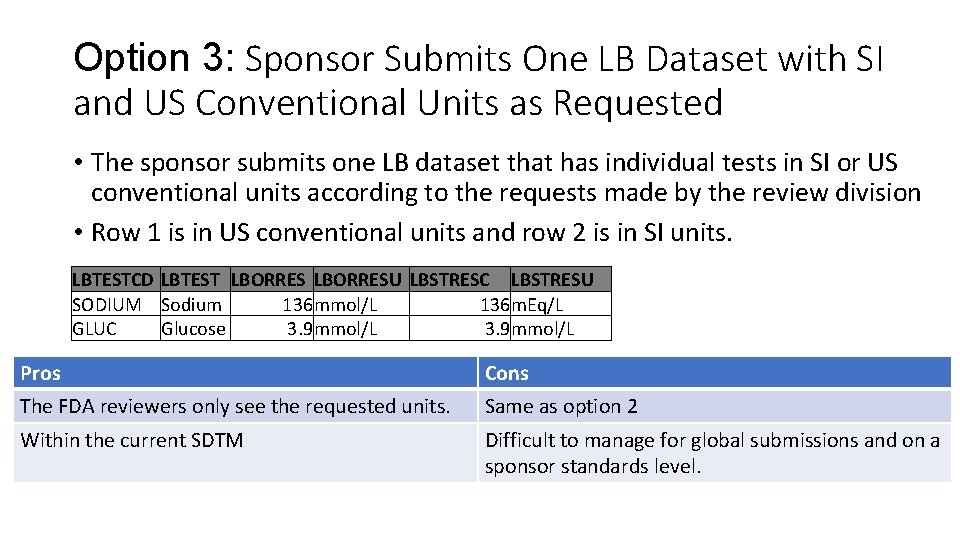
Option 3: Sponsor Submits One LB Dataset with SI and US Conventional Units as Requested • The sponsor submits one LB dataset that has individual tests in SI or US conventional units according to the requests made by the review division • Row 1 is in US conventional units and row 2 is in SI units. LBTESTCD LBTEST LBORRESU LBSTRESC LBSTRESU SODIUM Sodium 136 mmol/L 136 m. Eq/L GLUC Glucose 3. 9 mmol/L Pros Cons The FDA reviewers only see the requested units. Same as option 2 Within the current SDTM Difficult to manage for global submissions and on a sponsor standards level.
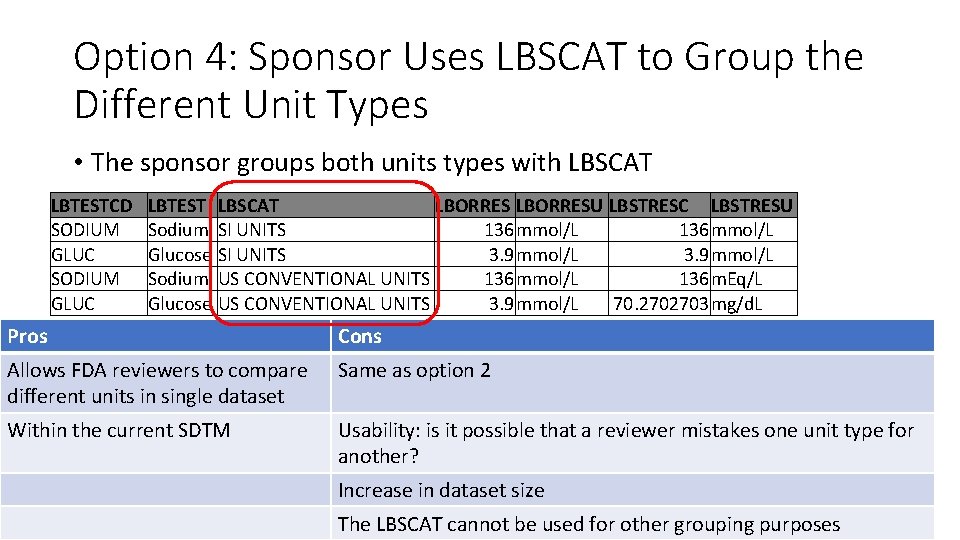
Option 4: Sponsor Uses LBSCAT to Group the Different Unit Types • The sponsor groups both units types with LBSCAT LBTESTCD SODIUM GLUC LBTEST LBSCAT LBORRESU LBSTRESC LBSTRESU Sodium SI UNITS 136 mmol/L Glucose SI UNITS 3. 9 mmol/L Sodium US CONVENTIONAL UNITS 136 mmol/L 136 m. Eq/L Glucose US CONVENTIONAL UNITS 3. 9 mmol/L 70. 2702703 mg/d. L Pros Cons Allows FDA reviewers to compare different units in single dataset Same as option 2 Within the current SDTM Usability: is it possible that a reviewer mistakes one unit type for another? Increase in dataset size The LBSCAT cannot be used for other grouping purposes
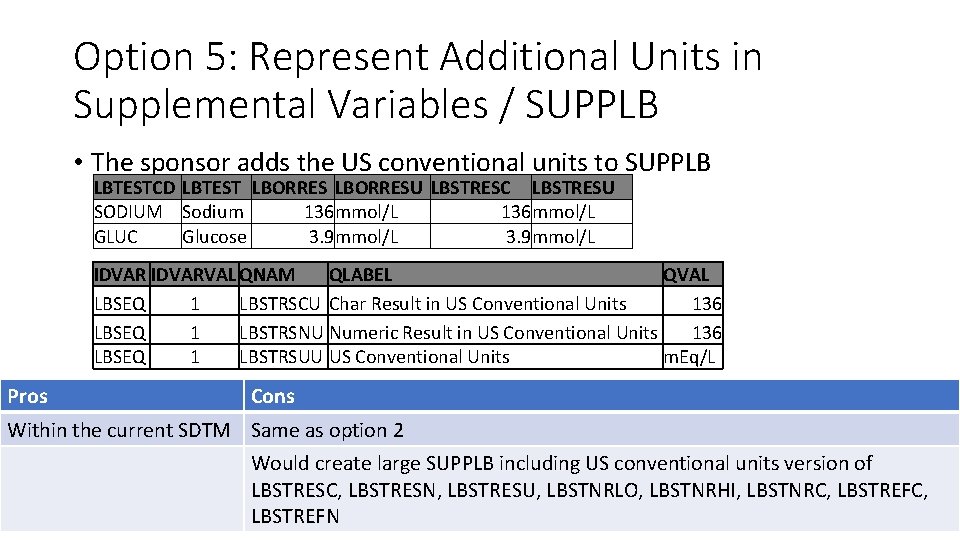
Option 5: Represent Additional Units in Supplemental Variables / SUPPLB • The sponsor adds the US conventional units to SUPPLB LBTESTCD LBTEST LBORRESU LBSTRESC LBSTRESU SODIUM Sodium 136 mmol/L GLUC Glucose 3. 9 mmol/L IDVARVAL QNAM QLABEL QVAL LBSEQ 1 LBSTRSCU Char Result in US Conventional Units 136 LBSEQ 1 LBSTRSNU Numeric Result in US Conventional Units 136 LBSEQ 1 LBSTRSUU US Conventional Units m. Eq/L Pros Cons Within the current SDTM Same as option 2 Would create large SUPPLB including US conventional units version of LBSTRESC, LBSTRESN, LBSTRESU, LBSTNRLO, LBSTNRHI, LBSTNRC, LBSTREFN
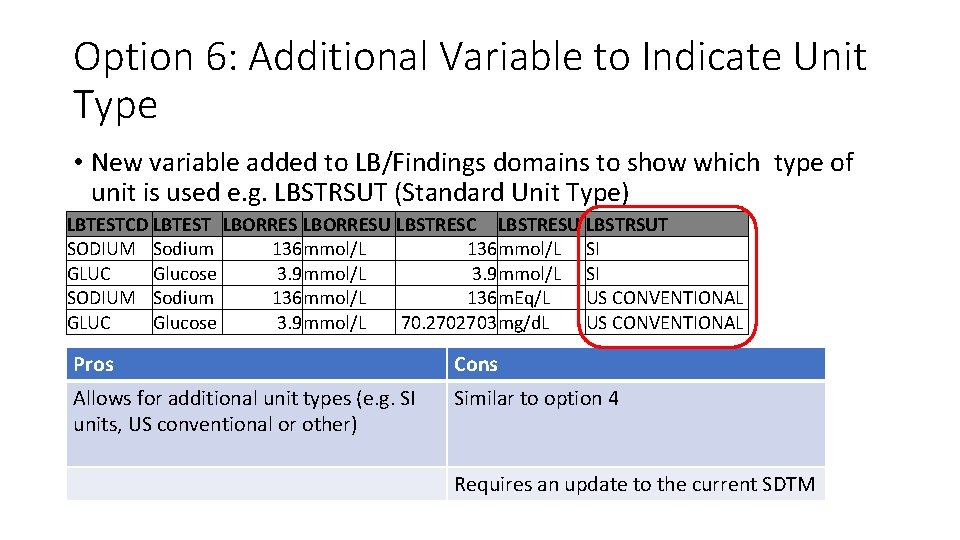
Option 6: Additional Variable to Indicate Unit Type • New variable added to LB/Findings domains to show which type of unit is used e. g. LBSTRSUT (Standard Unit Type) LBTESTCD LBTEST LBORRESU LBSTRESC LBSTRESU LBSTRSUT SODIUM Sodium 136 mmol/L SI GLUC Glucose 3. 9 mmol/L SI SODIUM Sodium 136 mmol/L 136 m. Eq/L US CONVENTIONAL GLUC Glucose 3. 9 mmol/L 70. 2702703 mg/d. L US CONVENTIONAL Pros Cons Allows for additional unit types (e. g. SI units, US conventional or other) Similar to option 4 Requires an update to the current SDTM
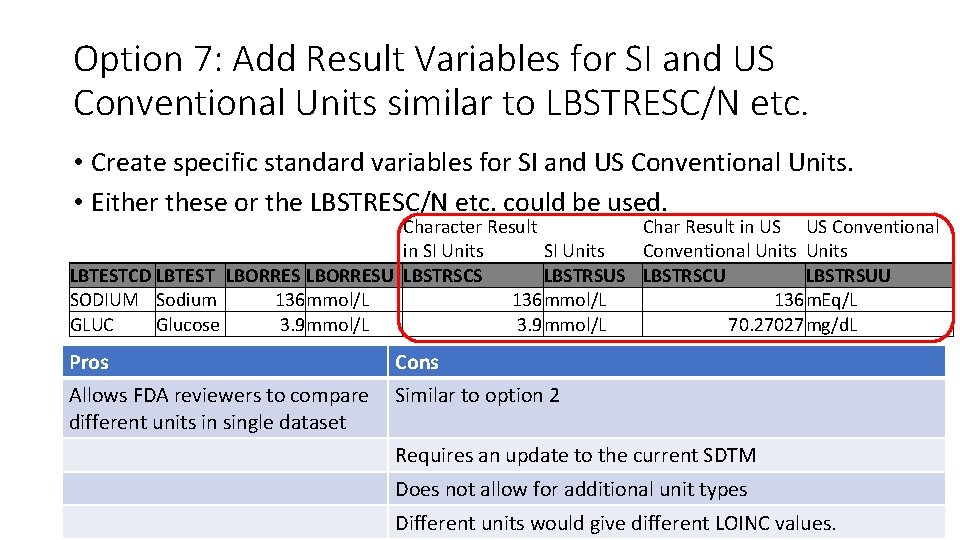
Option 7: Add Result Variables for SI and US Conventional Units similar to LBSTRESC/N etc. • Create specific standard variables for SI and US Conventional Units. • Either these or the LBSTRESC/N etc. could be used. Character Result Char Result in US US Conventional in SI Units Conventional Units LBTESTCD LBTEST LBORRESU LBSTRSCS LBSTRSUS LBSTRSCU LBSTRSUU SODIUM Sodium 136 mmol/L 136 m. Eq/L GLUC Glucose 3. 9 mmol/L 70. 27027 mg/d. L Pros Cons Allows FDA reviewers to compare different units in single dataset Similar to option 2 Requires an update to the current SDTM Does not allow for additional unit types Different units would give different LOINC values.
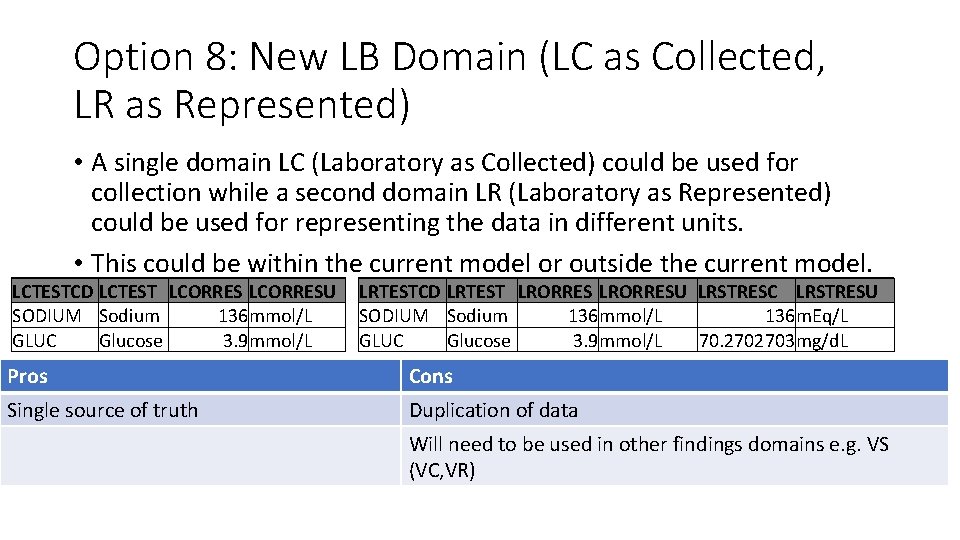
Option 8: New LB Domain (LC as Collected, LR as Represented) • A single domain LC (Laboratory as Collected) could be used for collection while a second domain LR (Laboratory as Represented) could be used for representing the data in different units. • This could be within the current model or outside the current model. LCTESTCD LCTEST LCORRESU SODIUM Sodium 136 mmol/L GLUC Glucose 3. 9 mmol/L LRTESTCD LRTEST LRORRESU LRSTRESC LRSTRESU SODIUM Sodium 136 mmol/L 136 m. Eq/L GLUC Glucose 3. 9 mmol/L 70. 2702703 mg/d. L Pros Cons Single source of truth Duplication of data Will need to be used in other findings domains e. g. VS (VC, VR)
Option 9: LAB Model • The CDISC LAB Model 1. 0. 1 section 3. 4. 13 Base Result provides an exchange standard for collected/reported, SI and US conventional units. • It is recognized that results may be preferred in more than one unit system so Reported, US Conventional and SI result fields have been provided. The Reported results are the results reported to the investigator sites. • The model is in XML and has naming conventions that do not conform to SDTM. • It is not known how many companies have implemented the standard Pros Cons Within the current SDTM Standard may not be widely adopted and require training Existing standard Provides the information in an XML format
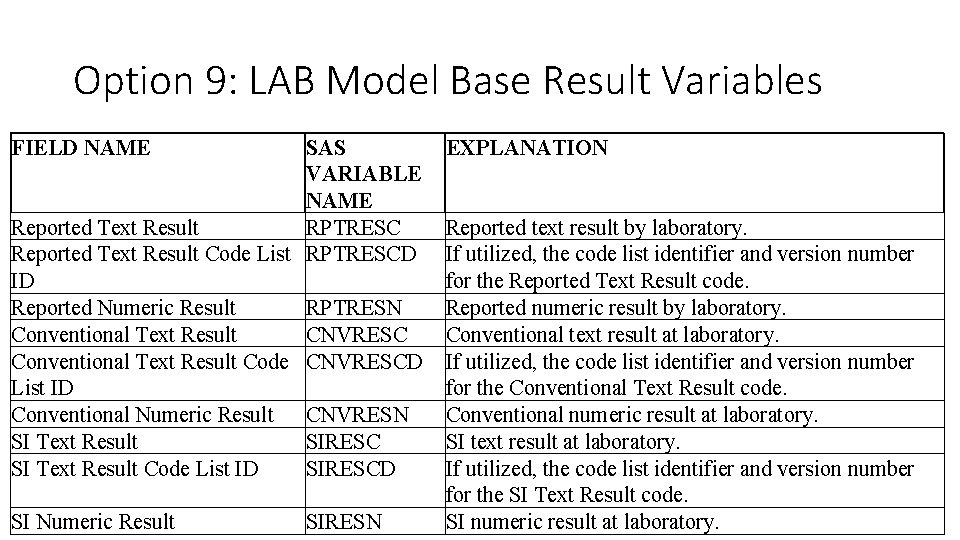
Option 9: LAB Model Base Result Variables FIELD NAME SAS VARIABLE NAME Reported Text Result RPTRESC Reported Text Result Code List RPTRESCD ID Reported Numeric Result RPTRESN Conventional Text Result CNVRESC Conventional Text Result Code CNVRESCD List ID Conventional Numeric Result CNVRESN SI Text Result SIRESC SI Text Result Code List ID SIRESCD EXPLANATION SI Numeric Result Reported text result by laboratory. If utilized, the code list identifier and version number for the Reported Text Result code. Reported numeric result by laboratory. Conventional text result at laboratory. If utilized, the code list identifier and version number for the Conventional Text Result code. Conventional numeric result at laboratory. SI text result at laboratory. If utilized, the code list identifier and version number for the SI Text Result code. SI numeric result at laboratory. SIRESN

Considerations
Lab Units Considerations • While working through the representation options it was noted that: 1. The conversion between reported and standard units is not described 2. The conversions may be carried out by SDTM programmers and not laboratory experts. • This could result in information being lost 3. Some labs may disagree on what the actual conversions are
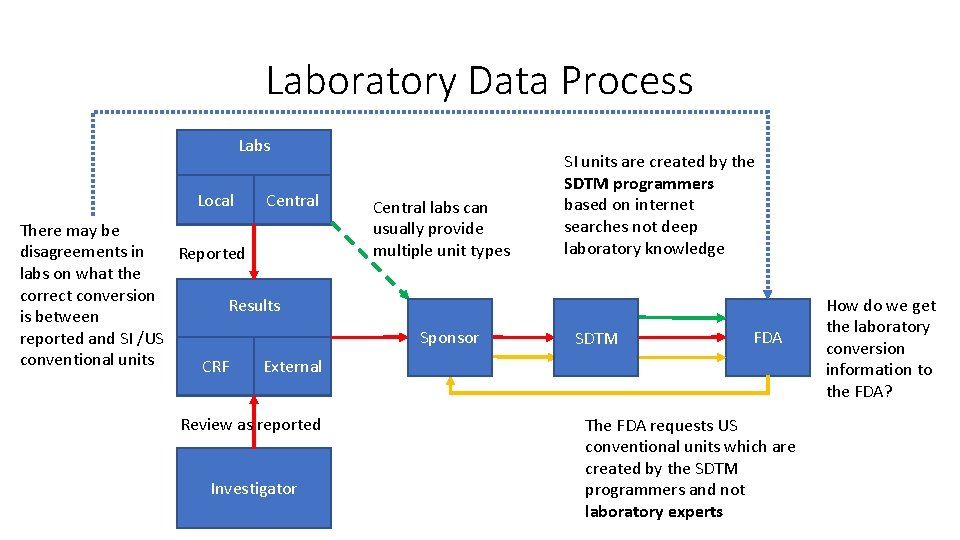
Laboratory Data Process Labs Local Central There may be disagreements in Reported labs on what the correct conversion Results is between reported and SI /US conventional units CRF External Review as reported Investigator Central labs can usually provide multiple unit types Sponsor SI units are created by the SDTM programmers based on internet searches not deep laboratory knowledge SDTM FDA The FDA requests US conventional units which are created by the SDTM programmers and not laboratory experts How do we get the laboratory conversion information to the FDA?
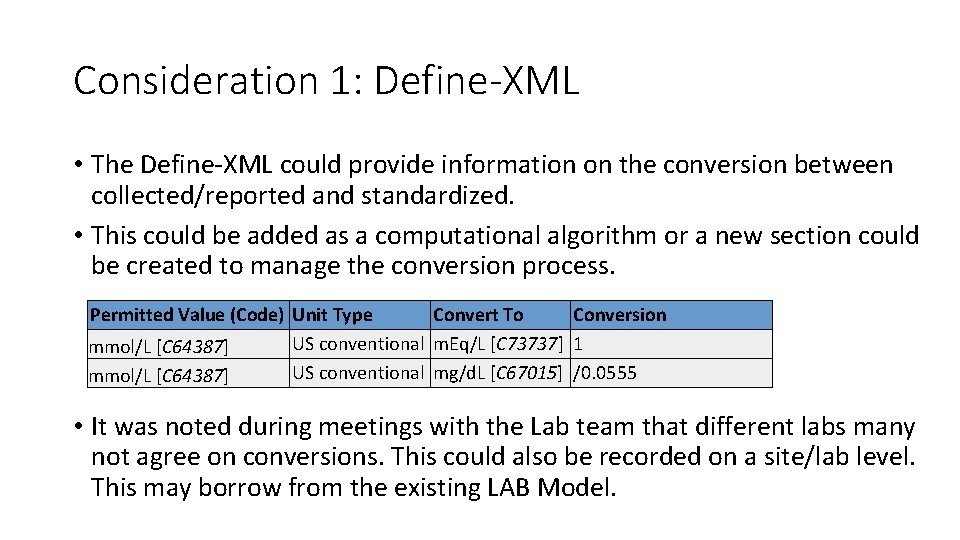
Consideration 1: Define-XML • The Define-XML could provide information on the conversion between collected/reported and standardized. • This could be added as a computational algorithm or a new section could be created to manage the conversion process. Permitted Value (Code) Unit Type Convert To Conversion US conventional m. Eq/L [C 73737] 1 mmol/L [C 64387] US conventional mg/d. L [C 67015] /0. 0555 mmol/L [C 64387] • It was noted during meetings with the Lab team that different labs many not agree on conversions. This could also be recorded on a site/lab level. This may borrow from the existing LAB Model.
Consideration 2: Ph. USE Lab Units Test Unit Plan • The Ph. USE Lab Units team created the Test Unit Plan (TUP). This is a communicative document between the FDA and sponsor to agree on a submission level, the units to be used for each lab test. • The TUP is an extension of the Study Data Standardization Plan. • Should this Ph. USE team restart?
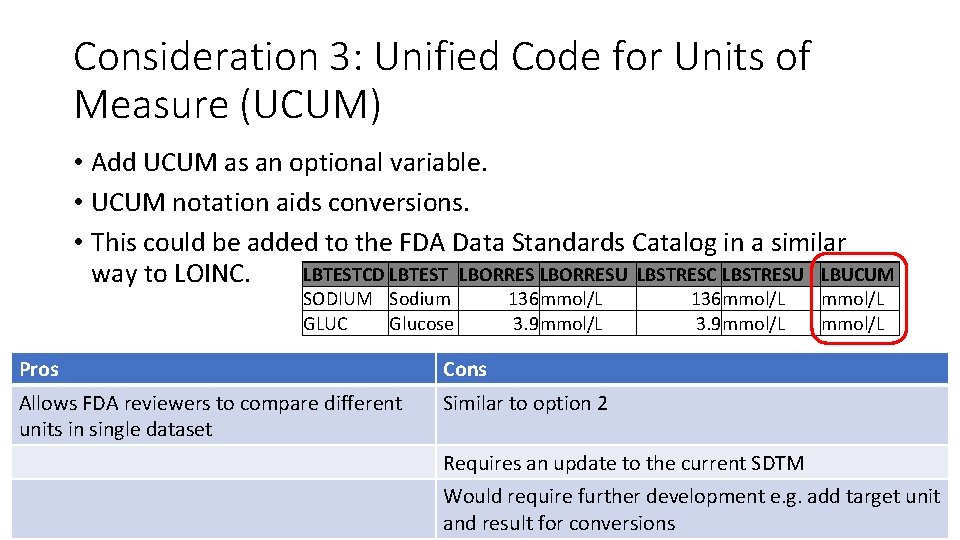
Consideration 3: Unified Code for Units of Measure (UCUM) • Add UCUM as an optional variable. • UCUM notation aids conversions. • This could be added to the FDA Data Standards Catalog in a similar LBTESTCD LBTEST LBORRESU LBSTRESC LBSTRESU LBUCUM way to LOINC. SODIUM Sodium GLUC Glucose 136 mmol/L 3. 9 mmol/L Pros Cons Allows FDA reviewers to compare different units in single dataset Similar to option 2 136 mmol/L 3. 9 mmol/L Requires an update to the current SDTM Would require further development e. g. add target unit and result for conversions

Consideration 4: CDISC/Lab Consortium/Other Lab Units Conversion Team • Should CDISC and/or a laboratory consortium maintain a set of conversion for specific laboratory values? • This was discussed in a Ph. USE group and the work needed to manage <10 tests took a number of days. • It may not be workable.

Open Questions • Is it possible for the FDA to speak with one voice on this topic or is this best left to the individual review divisions to decided which format is best for them? • Does the FDA keep a track of the number of times a review division requests US conventional units • If so does the FDA track which laboratory tests most frequently appear?
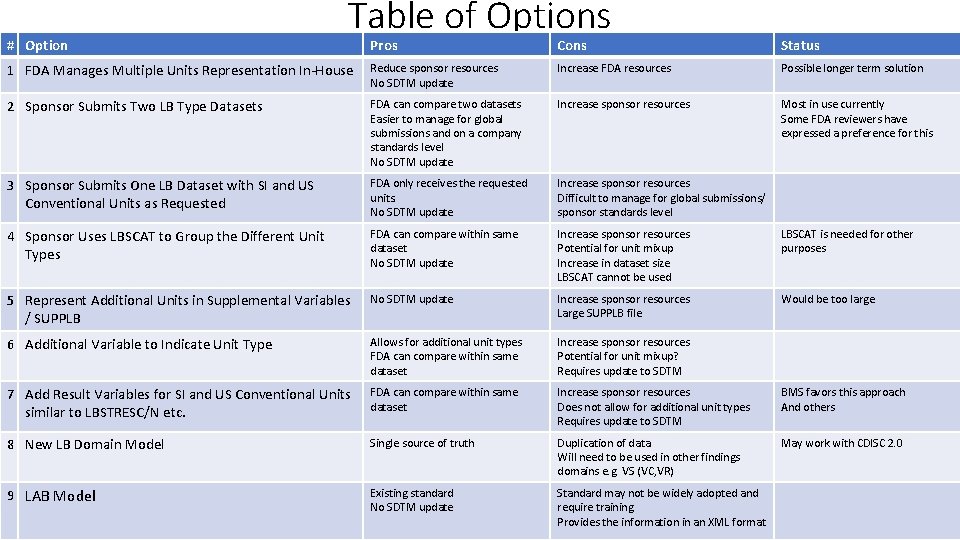
# Option Table of Options Pros Cons Status 1 FDA Manages Multiple Units Representation In-House Reduce sponsor resources No SDTM update Increase FDA resources Possible longer term solution 2 Sponsor Submits Two LB Type Datasets FDA can compare two datasets Easier to manage for global submissions and on a company standards level No SDTM update Increase sponsor resources Most in use currently Some FDA reviewers have expressed a preference for this 3 Sponsor Submits One LB Dataset with SI and US Conventional Units as Requested FDA only receives the requested units. No SDTM update Increase sponsor resources Difficult to manage for global submissions/ sponsor standards level 4 Sponsor Uses LBSCAT to Group the Different Unit Types FDA can compare within same dataset No SDTM update Increase sponsor resources Potential for unit mixup Increase in dataset size LBSCAT cannot be used LBSCAT is needed for other purposes 5 Represent Additional Units in Supplemental Variables / SUPPLB No SDTM update Increase sponsor resources Large SUPPLB file Would be too large 6 Additional Variable to Indicate Unit Type Allows for additional unit types FDA can compare within same dataset Increase sponsor resources Potential for unit mixup? Requires update to SDTM 7 Add Result Variables for SI and US Conventional Units similar to LBSTRESC/N etc. FDA can compare within same dataset Increase sponsor resources Does not allow for additional unit types Requires update to SDTM BMS favors this approach And others 8 New LB Domain Model Single source of truth Duplication of data Will need to be used in other findings domains e. g. VS (VC, VR) May work with CDISC 2. 0 9 LAB Model Existing standard No SDTM update Standard may not be widely adopted and require training Provides the information in an XML format

Table of Considerations # Consideration Comments 1 Define-XML Stores the SI and US Conventional Units and Conversions Moves information to Define-XML. How much is Define -XML used? 2 Ph. USE Lab Units Test Unit Plan Improves communication between FDA and sponsor 3 Unified Code for Units of Measure (UCUM) Could aid the conversions 4 CDISC/Lab Consortium/Other Lab Units Conversion Team Would be a large amount of work Status Will require implementation Lab team is already incorporating UCUM It does not solve all issues

Project Drivers • The FDA wants to provide reviewers/end users with units they are most proficient with • Lab Managers want to ensure that the conversions used reflect the underlying complexity of the lab tests • Standards teams want a single source or truth • Programmers want to know which conversions to use
Team Members Name Role Éanna Kiely Team Lead Alan Meier Programming Expert ? Team Email SDS eanna. kiely@clinbuild. com SDS alan. meier@cytel. com Lab Other?
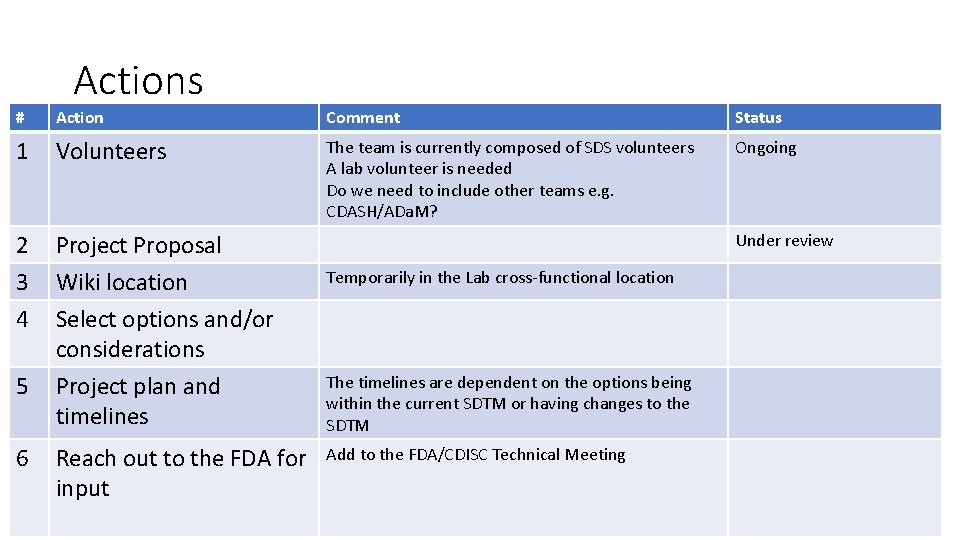
Actions # Action Comment Status 1 Volunteers The team is currently composed of SDS volunteers A lab volunteer is needed Do we need to include other teams e. g. CDASH/ADa. M? Ongoing 2 3 4 Project Proposal Wiki location Select options and/or considerations Project plan and timelines 5 6 Reach out to the FDA for input Under review Temporarily in the Lab cross-functional location The timelines are dependent on the options being within the current SDTM or having changes to the SDTM Add to the FDA/CDISC Technical Meeting

Questions Conversions from SI units to US conventional units taken from the AMA (American Medical Association) Manual of Style SI Conversion Calculator http: //www. amamanualofstyle. com/page/si-conversion-calculator
- Slides: 27