Multiple Ionization Energies and Atomic Radius of Ions


- Slides: 14

Multiple Ionization Energies and Atomic Radius of Ions

Recap! • What is the trend for atomic radius? • What is the trend for ionization energy? • What is the trend for electron affinity?

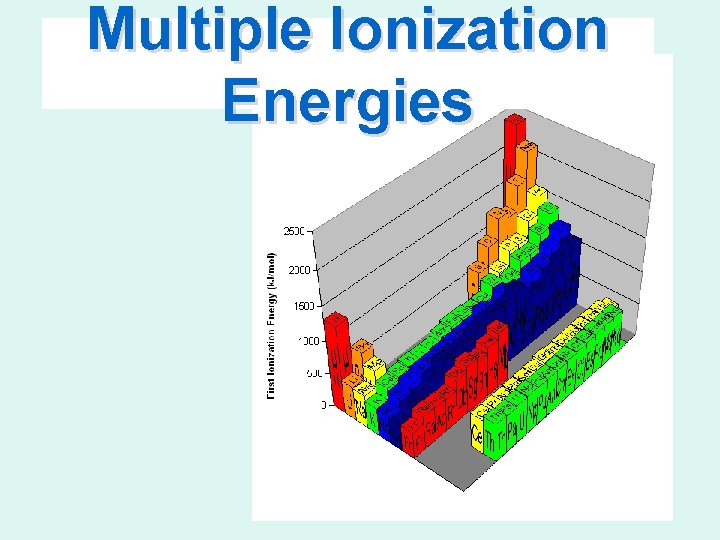
Multiple Ionization Energies

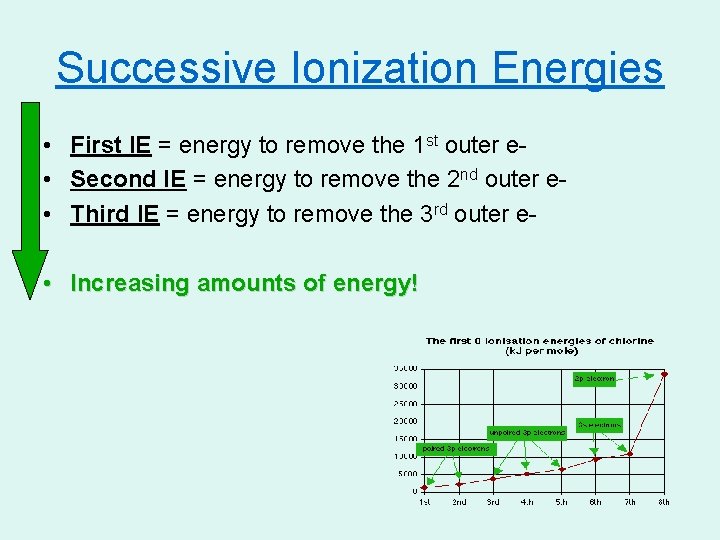
Successive Ionization Energies • First IE = energy to remove the 1 st outer e • Second IE = energy to remove the 2 nd outer e • Third IE = energy to remove the 3 rd outer e • Increasing amounts of energy!

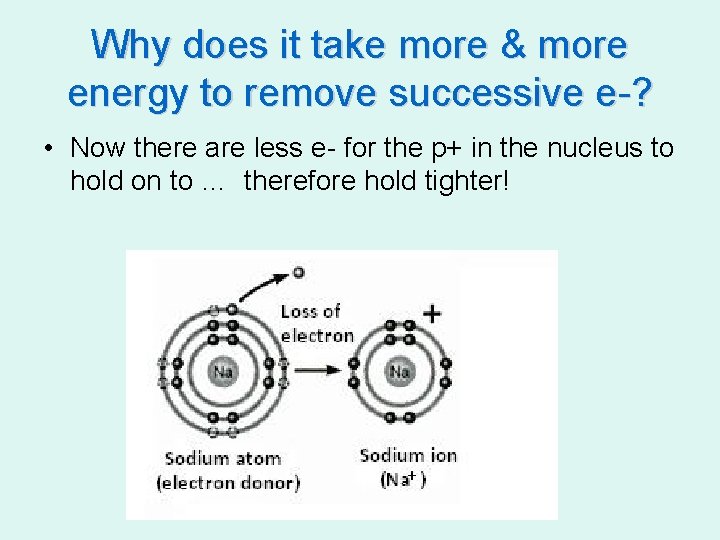
Why does it take more & more energy to remove successive e-? • Now there are less e- for the p+ in the nucleus to hold on to … therefore hold tighter!

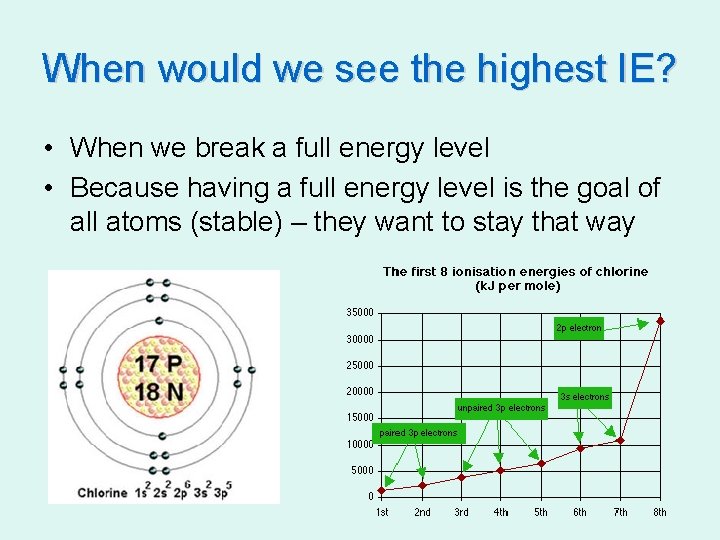
When would we see the highest IE? • When we break a full energy level • Because having a full energy level is the goal of all atoms (stable) – they want to stay that way

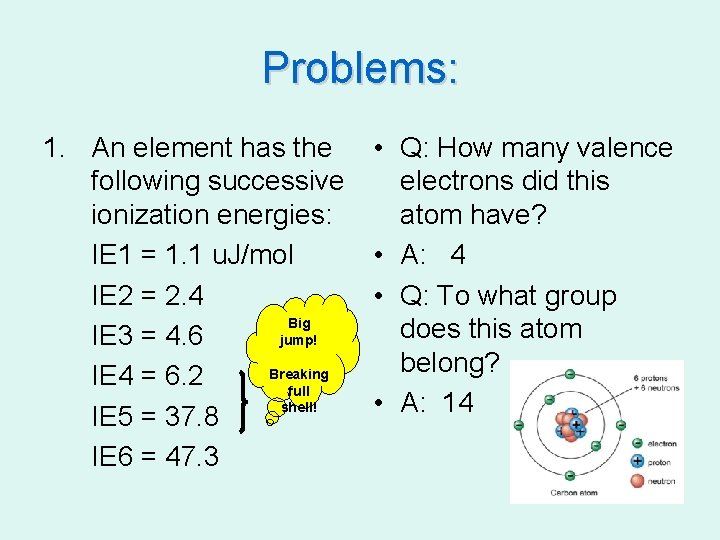
Problems: 1. An element has the following successive ionization energies: IE 1 = 1. 1 u. J/mol IE 2 = 2. 4 Big IE 3 = 4. 6 jump! Breaking IE 4 = 6. 2 full shell! IE 5 = 37. 8 IE 6 = 47. 3 • Q: How many valence electrons did this atom have? • A: 4 • Q: To what group does this atom belong? • A: 14

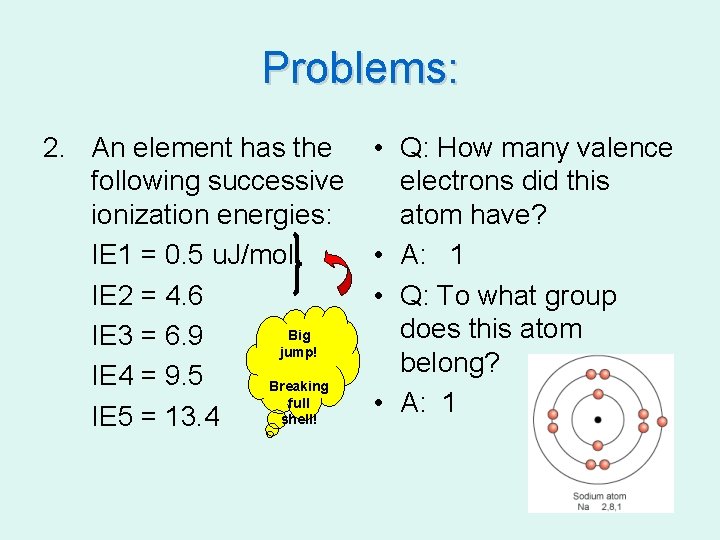
Problems: 2. An element has the following successive ionization energies: IE 1 = 0. 5 u. J/mol IE 2 = 4. 6 Big IE 3 = 6. 9 jump! IE 4 = 9. 5 Breaking full shell! IE 5 = 13. 4 • Q: How many valence electrons did this atom have? • A: 1 • Q: To what group does this atom belong? • A: 1

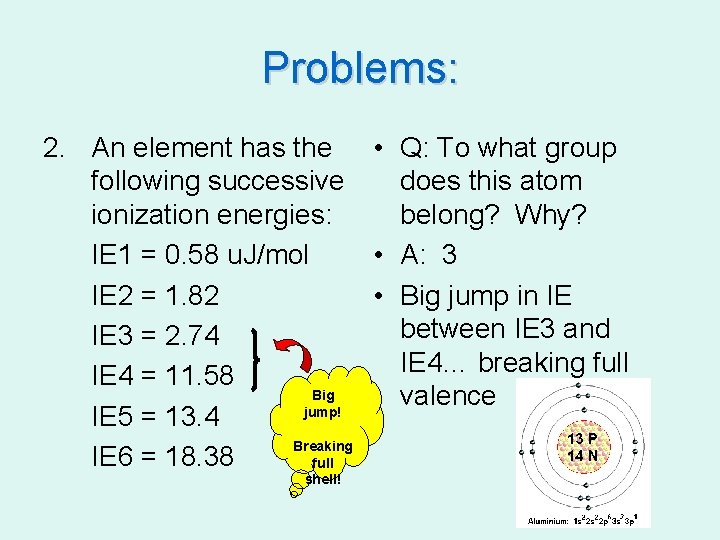
Problems: 2. An element has the • Q: To what group following successive does this atom ionization energies: belong? Why? IE 1 = 0. 58 u. J/mol • A: 3 IE 2 = 1. 82 • Big jump in IE between IE 3 and IE 3 = 2. 74 IE 4… breaking full IE 4 = 11. 58 Big valence jump! IE 5 = 13. 4 Breaking IE 6 = 18. 38 full shell!

ATOMIC RADIUS OF IONS

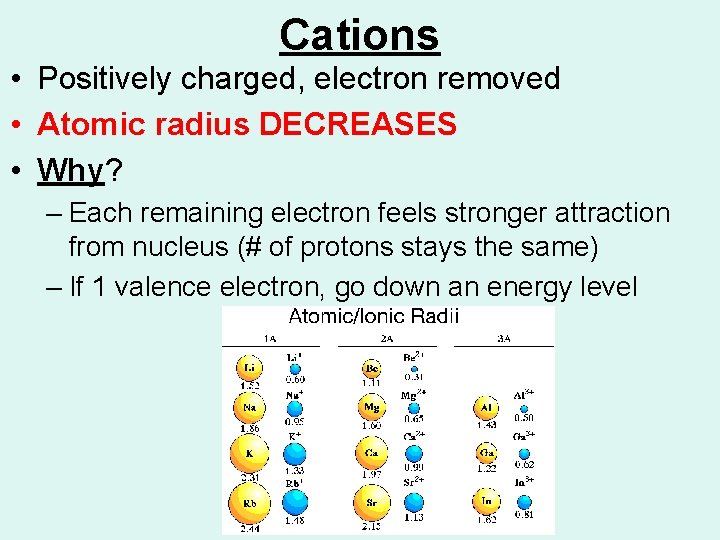
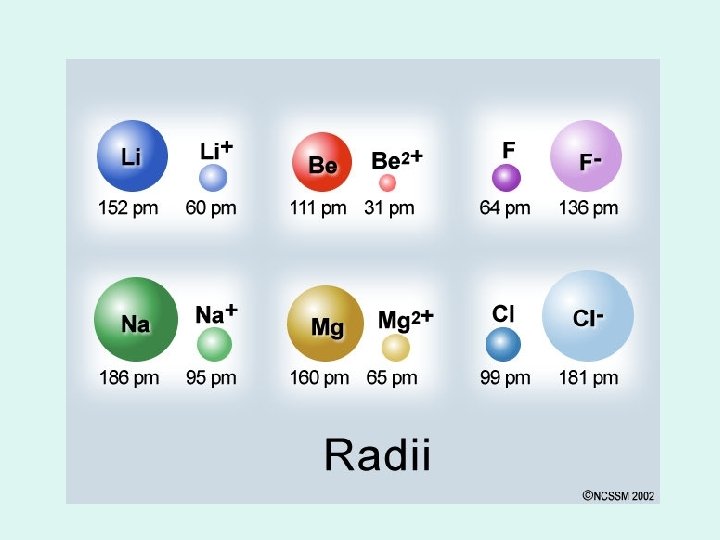
Cations • Positively charged, electron removed • Atomic radius DECREASES • Why? – Each remaining electron feels stronger attraction from nucleus (# of protons stays the same) – If 1 valence electron, go down an energy level

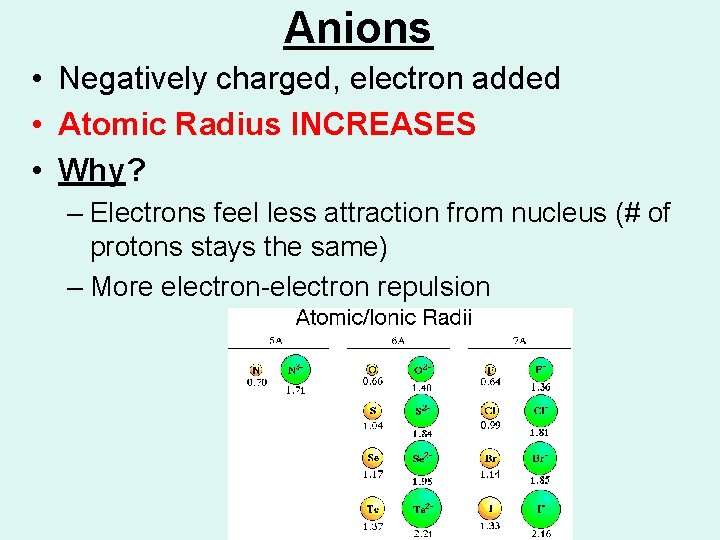
Anions • Negatively charged, electron added • Atomic Radius INCREASES • Why? – Electrons feel less attraction from nucleus (# of protons stays the same) – More electron-electron repulsion


HOMEWORK • Read pg 49 -58 • Questions – pg. 52 #7 (every other one) – Pg. 55 #8 -9 (every other one) – Pg. 60 #1, 2 -4 (every other one) • Review Chapter 2 • Chapter 2 QUIZ on THURSDAY