Multiple Endpoint Testing in Clinical Trials Some Issues















- Slides: 30

Multiple Endpoint Testing in Clinical Trials – Some Issues & Considerations Mohammad Huque, Ph. D. Division of Biometrics III/Office of Biostatistics/OPa. SS/CDER/FDA 2005 Industry/FDA Workshop, Washington. DC 2/28/2021

Disclaimer • Views expressed here is that of the presenter and not necessarily of the FDA 2/28/2021

Sources of Multiplicity in Clinical Trials • Multiple endpoints • • • Multiple comparisons Interim analysis Subgroup analysis Selection of covariates in an analysis model Others 2/28/2021

OUTLINE 1. 2. 3. 4. 5. Type I error concept and type I error control when testing for multiple endpoints. Complexities? Multiple endpoints are often triaged into primary, secondary and other types of endpoints. Reasons for doing so and how these endpoints are tested? Sequential testing of endpoints - no alpha adjustment is needed. Issues and fixes? Some trials require that 2 or more endpoints must show effects for clinical evidence. Reasons for doing so and consequences? Composite endpoints. Underlying concepts and complexities? 2/28/2021

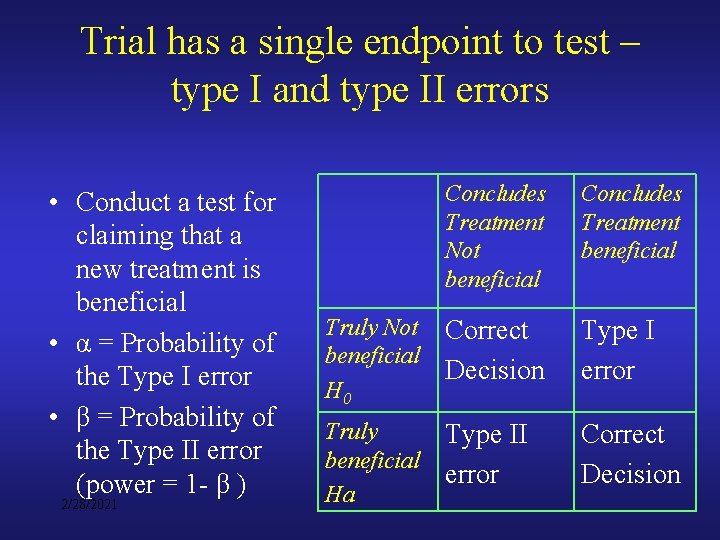
Trial has a single endpoint to test – type I and type II errors • Conduct a test for claiming that a new treatment is beneficial • α = Probability of the Type I error • β = Probability of the Type II error (power = 1 - β ) 2/28/2021 Concludes Treatment Not beneficial Concludes Treatment beneficial Truly Not beneficial H 0 Correct Decision Type I error Truly beneficial Ha Type II error Correct Decision

Trial has multiple endpoints to test • Consider a two arm superiority trial, a test treatment versus a control Endpoints: y 1, y 2, …, y. K Multiple Null Hypotheses: F = {H 01, H 02, …, H 0 K} H 0 j: δj = 0, Haj δj ≠ 0, j =1, …, K 2/28/2021

Trial has multiple endpoints to test • Two scenarios: (A) In the family F all are true null hypotheses (B) Some may be true null hypotheses, and some may be false null hypotheses, but their true state are unknown. 2/28/2021

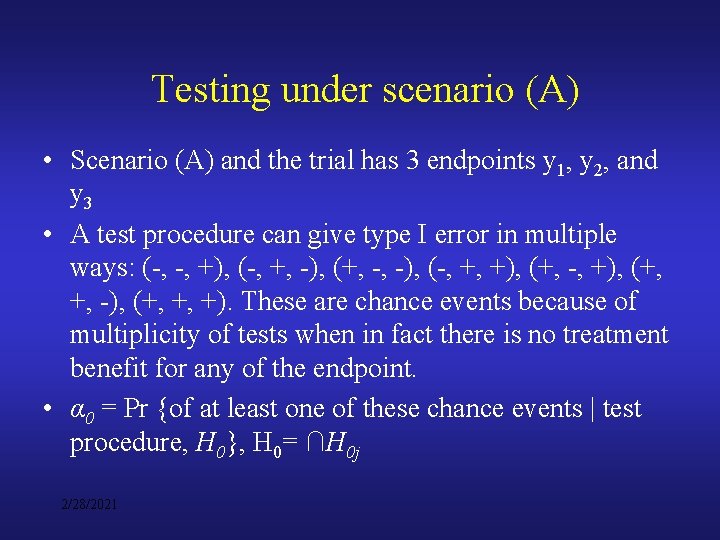
Testing under scenario (A) • Scenario (A) and the trial has 3 endpoints y 1, y 2, and y 3 • A test procedure can give type I error in multiple ways: (-, -, +), (-, +, -), (+, -, -), (-, +, +), (+, -, +), (+, +, -), (+, +, +). These are chance events because of multiplicity of tests when in fact there is no treatment benefit for any of the endpoint. • α 0 = Pr {of at least one of these chance events | test procedure, H 0}, H 0= ∩H 0 j 2/28/2021

Testing under scenario (A) • α 0 is called global alpha (or overall alpha). Also, called the familywise type I error rate (FWER) under H 0, where • H 0= ∩H 0 j is the global null hypothesis. • A test procedure for testing H 0 is called a global test procedure 2/28/2021

Global Test procedures • Useful for non-specific global claims. Difficulty in interpreting the result. Type I error rate can remain inflated for specific claims. • Examples: Simes test, O’Brien’s OLS/GLS tests, Hotelling’s T 2 test (Sankoh et al, DIA Jr. , 1999) 2/28/2021

Testing under scenario (B) • Some of the null hypotheses F = {H 01, H 02, …, H 0 K} may be true null hypotheses and some be false, but its not known which ones are which. • Question: Is there a treatment effect specifically for the endpoint y 1? • For answering this question, the null hypothesis is not a single null hypothesis like a global null hypothesis, rather it is a class of null hypothesis configurations in which there is no treatment effect for y 1, and all possible scenarios for treatment effects for the remaining endpoints y 2, …, y. K 2/28/2021

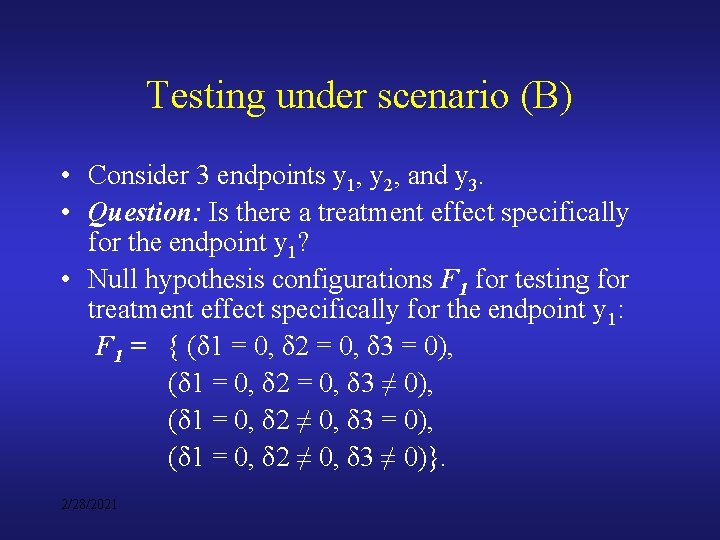
Testing under scenario (B) • Consider 3 endpoints y 1, y 2, and y 3. • Question: Is there a treatment effect specifically for the endpoint y 1? • Null hypothesis configurations F 1 for testing for treatment effect specifically for the endpoint y 1: F 1 = { (δ 1 = 0, δ 2 = 0, δ 3 = 0), (δ 1 = 0, δ 2 = 0, δ 3 ≠ 0), (δ 1 = 0, δ 2 ≠ 0, δ 3 = 0), (δ 1 = 0, δ 2 ≠ 0, δ 3 ≠ 0)}. 2/28/2021

Control of FWER • Weak control (two types) – Control FWER only under the global null configuration • Strong control – Control FWER under all null configurations – Specificity property -- useful for making specific claims. – Examples of methods: Bonferroni, Holm, Hochberg*, closed statistical tests, and other methods *with some caveats 2/28/2021

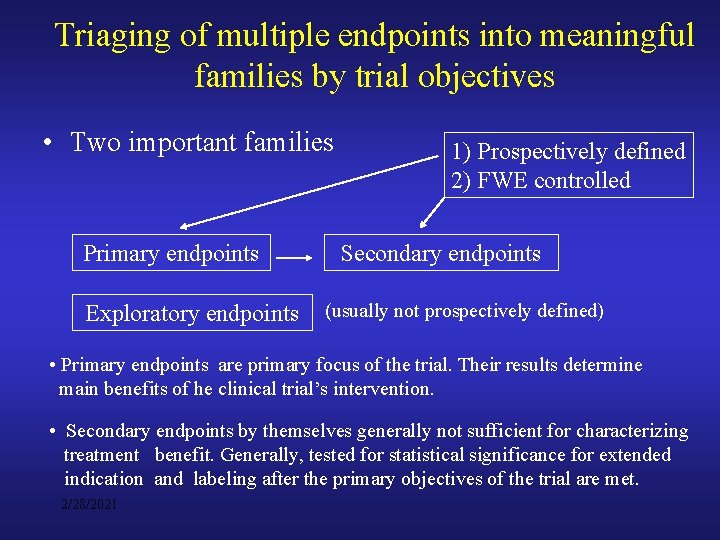
Triaging of multiple endpoints into meaningful families by trial objectives • Two important families Primary endpoints Exploratory endpoints 1) Prospectively defined 2) FWE controlled Secondary endpoints (usually not prospectively defined) • Primary endpoints are primary focus of the trial. Their results determine main benefits of he clinical trial’s intervention. • Secondary endpoints by themselves generally not sufficient for characterizing treatment benefit. Generally, tested for statistical significance for extended indication and labeling after the primary objectives of the trial are met. 2/28/2021

Statistical methods • Prospective alpha allocation schemes (PAAS) – Moyé (2000) – Spend alpha 1 for the primary endpoints and the remaining alpha for the secondary endpoints FWER is controlled 2/28/2021

Statistical methods • Parallel gatekeeping strategies for clinical trials – – Dmitrienko-Offen-Westfall (SM 2003) – Chen-Luo-Capizzi (SM 2005) • Allows testing of secondary endpoints when at least one of the primary endpoints exhibits a statistically significant result • These methods controls FWER for both the primary and secondary endpoints in the 2/28/2021 strong sense.

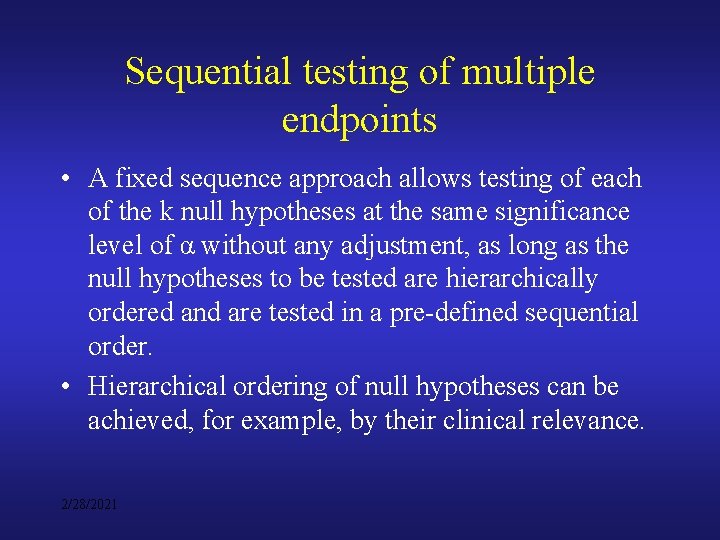
Sequential testing of multiple endpoints • A fixed sequence approach allows testing of each of the k null hypotheses at the same significance level of α without any adjustment, as long as the null hypotheses to be tested are hierarchically ordered and are tested in a pre-defined sequential order. • Hierarchical ordering of null hypotheses can be achieved, for example, by their clinical relevance. 2/28/2021

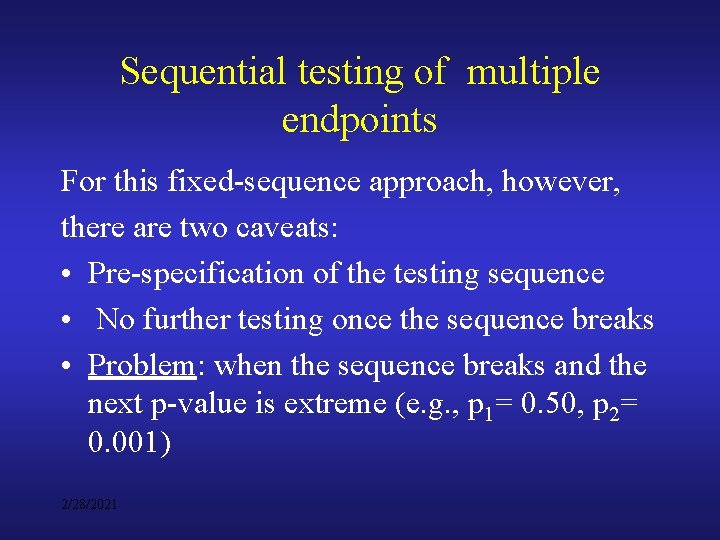
Sequential testing of multiple endpoints For this fixed-sequence approach, however, there are two caveats: • Pre-specification of the testing sequence • No further testing once the sequence breaks • Problem: when the sequence breaks and the next p-value is extreme (e. g. , p 1= 0. 50, p 2= 0. 001) 2/28/2021

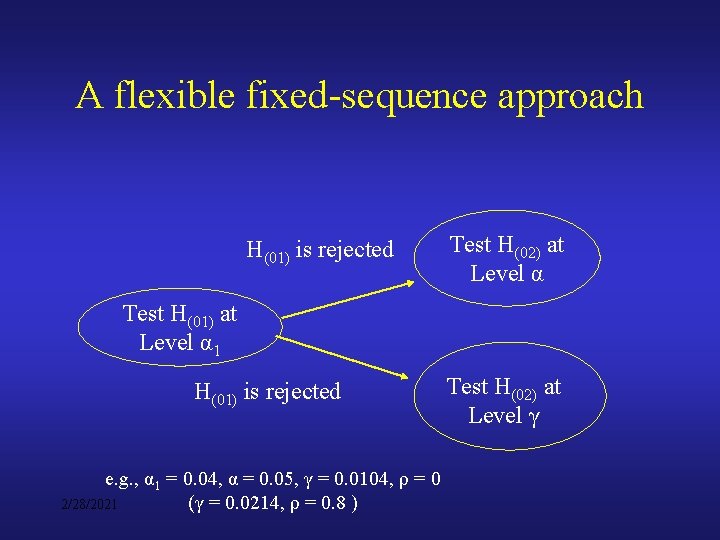
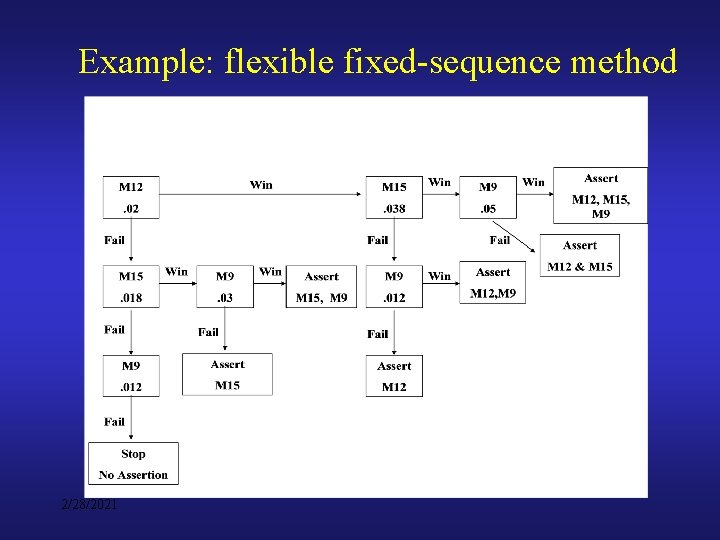
A flexible fixed-sequence approach H(01) is rejected Test H(02) at Level α Test H(01) at Level α 1 H(01) is rejected e. g. , α 1 = 0. 04, α = 0. 05, γ = 0. 0104, ρ = 0 (γ = 0. 0214, ρ = 0. 8 ) 2/28/2021 Test H(02) at Level γ

Example: flexible fixed-sequence method 2/28/2021

Some trials require that 2 or more endpoints must show effects Examples: • Alzheimer trial – (win on ADAS-Cognitive Sub-scale) and (win on Clinician’s Interview Based Impression of Change) • Many other examples (Ph. RMA draft paper) Main Reason: • Clinical expectations of the desired clinical benefit (concept beyond statistics) 2/28/2021

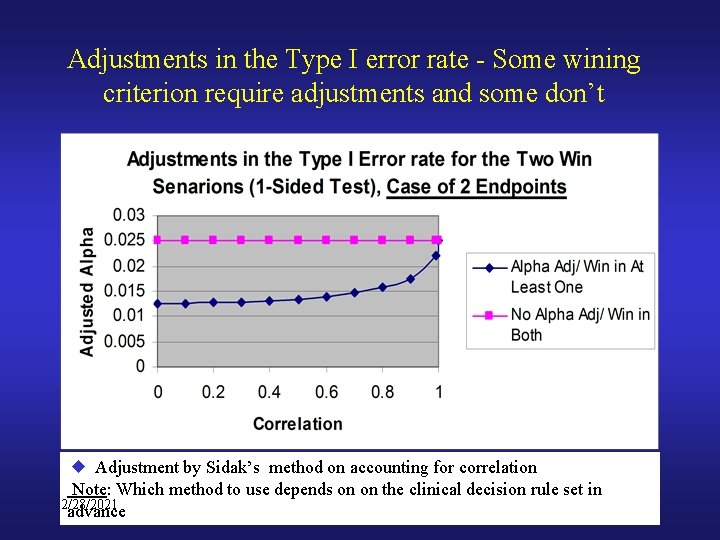
Adjustments in the Type I error rate - Some wining criterion require adjustments and some don’t Adjustment by Sidak’s method on accounting for correlation Note: Which method to use depends on on the clinical decision rule set in 2/28/2021 advance

Power Comparison Case of K=2 endpoints: 2/28/2021

Loss in Power when win in all endpoints K=# of endpoints 2/28/2021

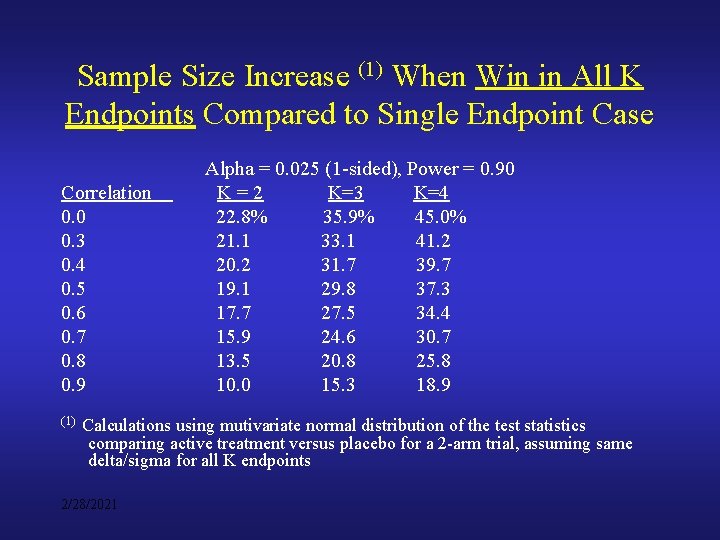
Sample Size Increase (1) When Win in All K Endpoints Compared to Single Endpoint Case Correlation 0. 0 0. 3 0. 4 0. 5 0. 6 0. 7 0. 8 0. 9 (1) Alpha = 0. 025 (1 -sided), Power = 0. 90 K=2 K=3 K=4 22. 8% 35. 9% 45. 0% 21. 1 33. 1 41. 2 20. 2 31. 7 39. 7 19. 1 29. 8 37. 3 17. 7 27. 5 34. 4 15. 9 24. 6 30. 7 13. 5 20. 8 25. 8 10. 0 15. 3 18. 9 Calculations using mutivariate normal distribution of the test statistics comparing active treatment versus placebo for a 2 -arm trial, assuming same delta/sigma for all K endpoints 2/28/2021

Composite Endpoints Two types • Total score or index based on a rating scale, e. g. , HAMD totals in depression trials, ACR 20/ACR 70 in rheumatoid arthritis trials Issues: validity and reliability 2/28/2021

Composite Endpoints Another Type • Composite endpoint is defined in terms of the time to the first “event”, where event is one of several possible event types LIFE study: Composite of cardiovascular death, stroke and myocardial infraction events. 2/28/2021

Composite Endpoint Issues Life Study The Composite endpoint was significantly positive. However, analysis of the first events by individual components and sub-composite endpoints indicate overall composite result mainly due to reduction in fatal and non-fatal stroke. Issue: How to interpret composite endpoint results? How to characterize benefits in terms of the component endpoints? 2/28/2021

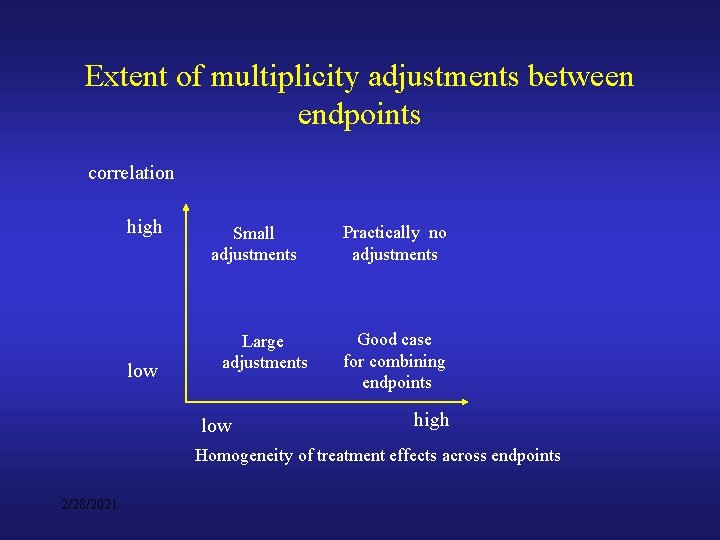
Extent of multiplicity adjustments between endpoints correlation high low Small adjustments Large adjustments low Practically no adjustments Good case for combining endpoints high Homogeneity of treatment effects across endpoints 2/28/2021

Concluding Remarks • For endpoint specific claims – strong control of the type I error is needed • Parallel gate-keeping strategies can be used for the primary and secondary endpoint claims • Flexible sequential test procedure can be used to gain power of the test • There is a scientific basis when a reasonable clinical decision rule asks for statistically significant efficacy results in more than 1 endpoint – issue of loss of power? • When 4 or more endpoints included as primary (e. g. , arthritis trials), and homogeneity of treatment effects acress endpoints is expected - a composite or responder endpoint approach will be effective. 2/28/2021