Multiple Choice definitions chem reaction catalyst coefficient subscript

Multiple Choice definitions: chem reaction, catalyst, coefficient, subscript skeleton eq vs balanced eq types of reactions Open Ended 5 balancing 2 word equations 5 predict the products
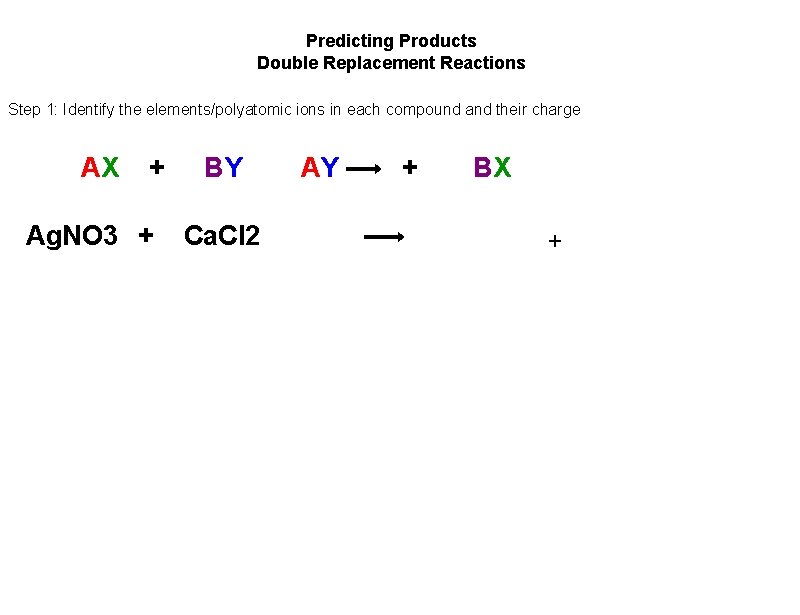
Predicting Products Double Replacement Reactions Step 1: Identify the elements/polyatomic ions in each compound and their charge AX + Ag. NO 3 + BY Ca. Cl 2 AY + BX +
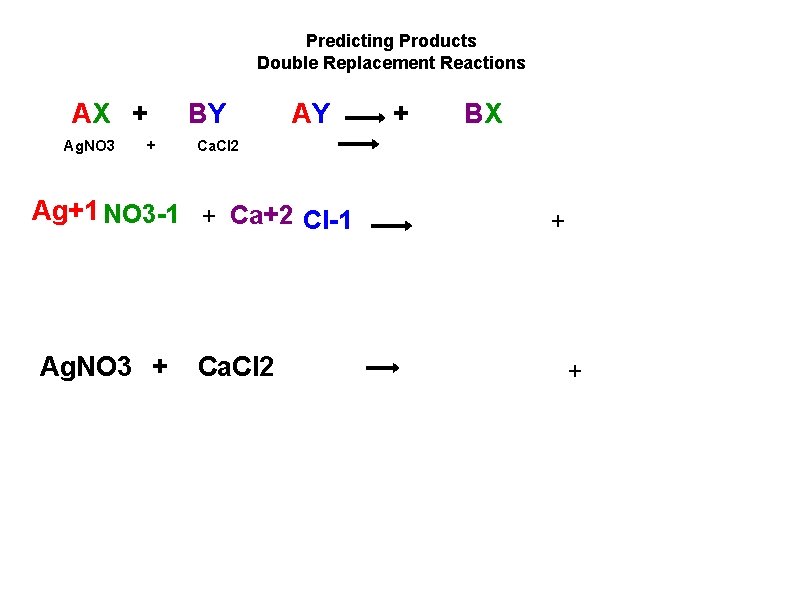
Predicting Products Double Replacement Reactions AX + Ag. NO 3 + BY AY BX Ca. Cl 2 Ag+1 NO 3 1 + Ca+2 Cl 1 Ag. NO 3 + + Ca. Cl 2 + +
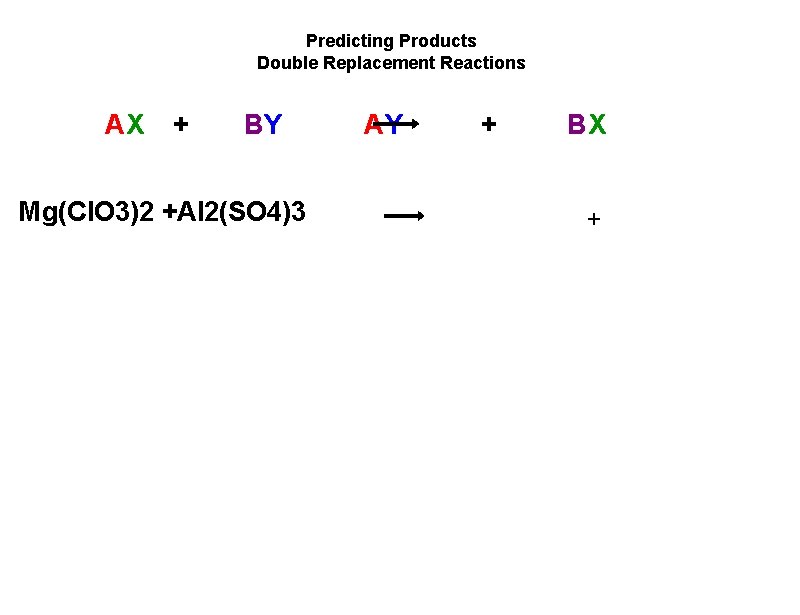
Predicting Products Double Replacement Reactions AX + BY Mg(Cl. O 3)2 +Al 2(SO 4)3 AY + BX +
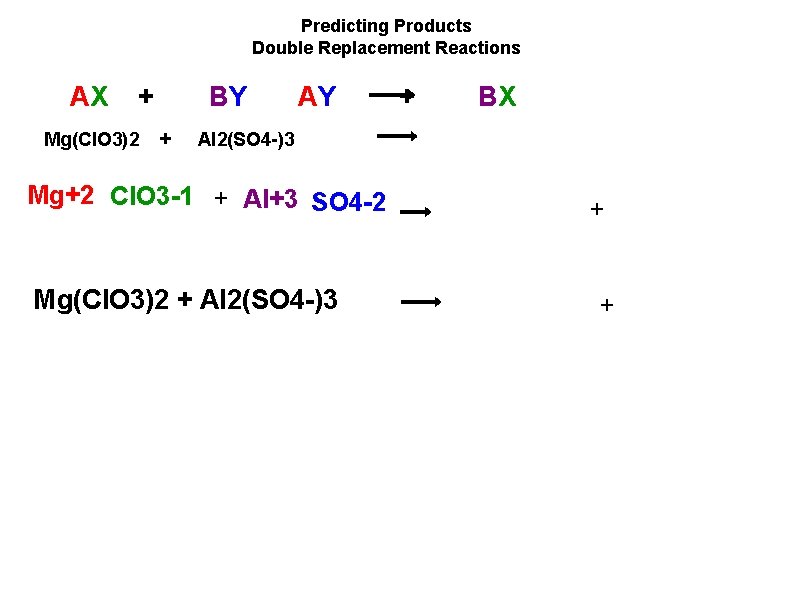
Predicting Products Double Replacement Reactions AX + Mg(Cl. O 3)2 BY + AY + BX Al 2(SO 4 )3 Mg+2 Cl. O 3 1 + Al+3 SO 4 2 Mg(Cl. O 3)2 + Al 2(SO 4 )3 + +
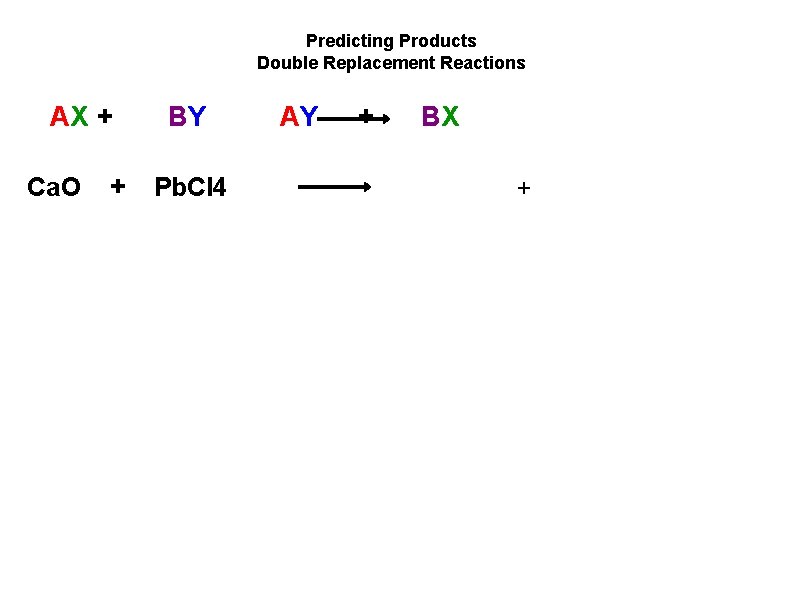
Predicting Products Double Replacement Reactions AX + Ca. O + BY Pb. Cl 4 AY + BX +
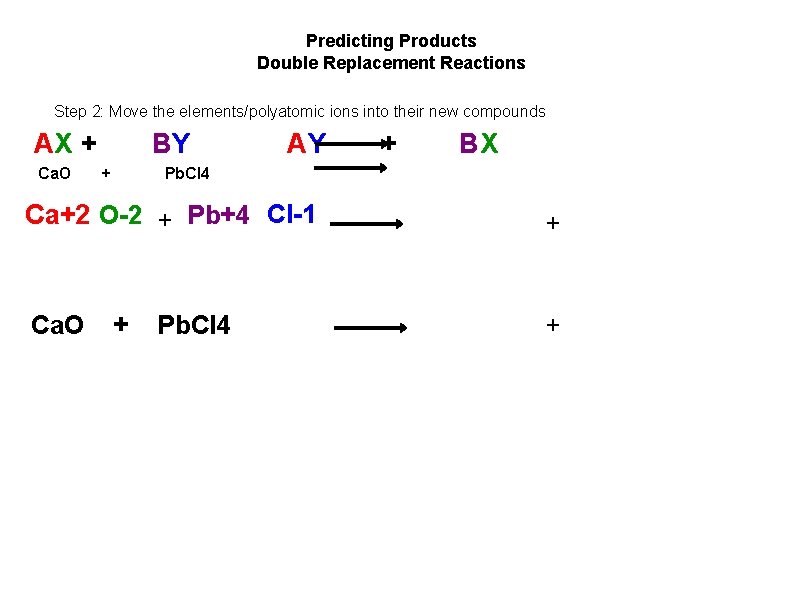
Predicting Products Double Replacement Reactions Step 2: Move the elements/polyatomic ions into their new compounds AX + Ca. O BY + AY + BX Pb. Cl 4 Ca+2 O 2 + Pb+4 Cl 1 + Ca. O + + Pb. Cl 4
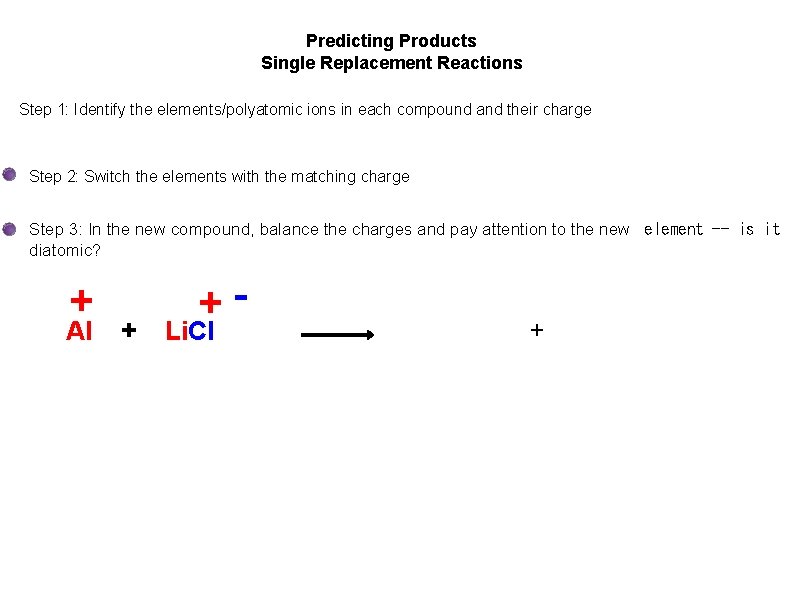
Predicting Products Single Replacement Reactions Step 1: Identify the elements/polyatomic ions in each compound and their charge Step 2: Switch the elements with the matching charge Step 3: In the new compound, balance the charges and pay attention to the new element -- is it diatomic? + Al + + Li. Cl +
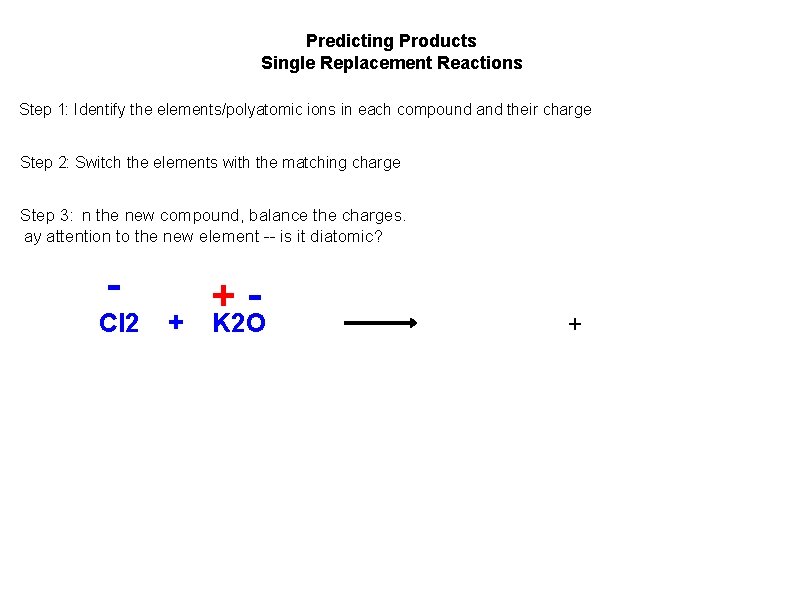
Predicting Products Single Replacement Reactions Step 1: Identify the elements/polyatomic ions in each compound and their charge Step 2: Switch the elements with the matching charge Step 3: n the new compound, balance the charges. ay attention to the new element -- is it diatomic? Cl 2 + + K 2 O +
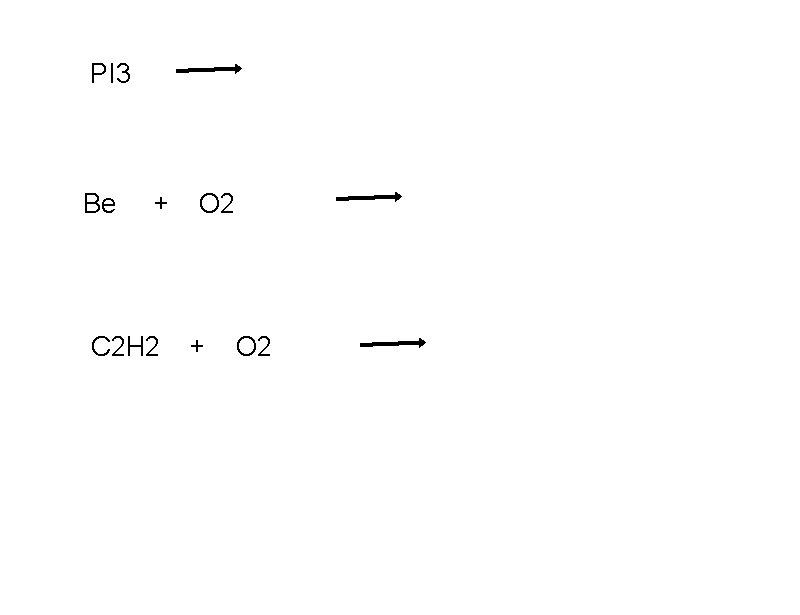
PI 3 Be + C 2 H 2 O 2 + O 2
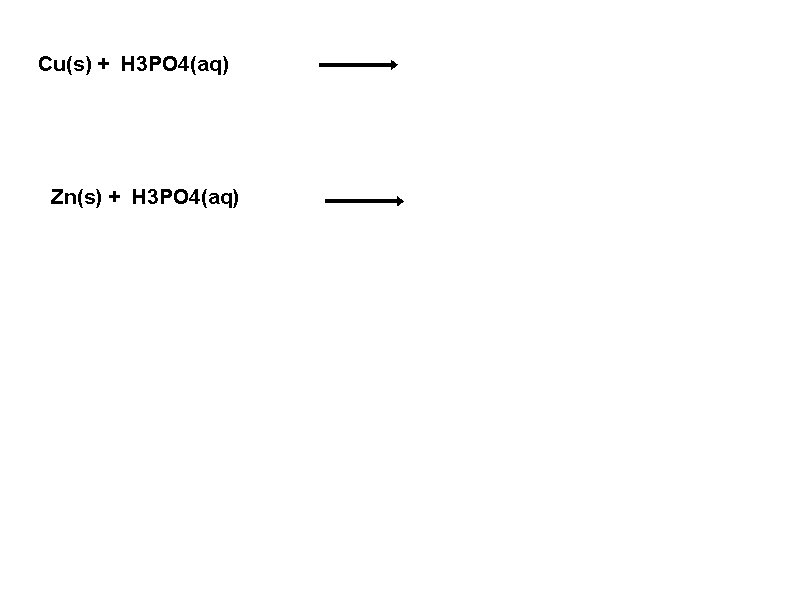
Cu(s) + H 3 PO 4(aq) Zn(s) + H 3 PO 4(aq)




- Slides: 15