Multiphysics Galvanic Corrosion Analysis for CarbonAluminium Structures under
Multi-physics Galvanic Corrosion Analysis for Carbon-Aluminium Structures under Saltwater Film Yuki ONISHI, Koichi MASUYA, Kenji AMAYA Tokyo Institute of Technology, Japan P. 1 FEOFS 2016 P. 1
Background n The multi-material design of vehicles is recently in progress in order to reduce the weight. n Especially, carbon fiber reinforced plastics (CFRP) and aluminium alloys (Al) are coming into use. n As a result, galvanic corrosion occurs around the joint parts of different materials under saltwater film (e. g. , seawater, solution of snow melting agents, etc. ). Fig. multi-material designed vehicle BMW Japan Corp. http: //www. bmw. co. jp/jp/ja/ insights/corporation/bmwi/ concept. html FEOFS 2016 P. 2
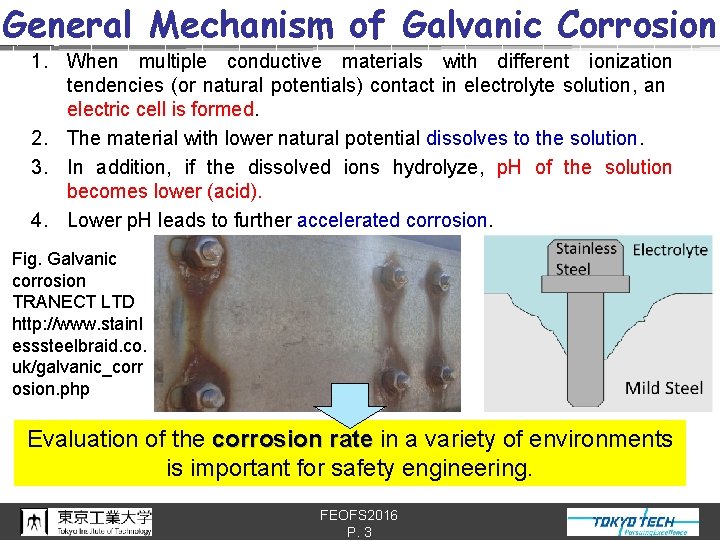
General Mechanism of Galvanic Corrosion 1. When multiple conductive materials with different ionization tendencies (or natural potentials) contact in electrolyte solution, an electric cell is formed. 2. The material with lower natural potential dissolves to the solution. 3. In addition, if the dissolved ions hydrolyze, p. H of the solution becomes lower (acid). 4. Lower p. H leads to further accelerated corrosion. Fig. Galvanic corrosion TRANECT LTD http: //www. stainl esssteelbraid. co. uk/galvanic_corr osion. php Evaluation of the corrosion rate in a variety of environments is important for safety engineering. FEOFS 2016 P. 3
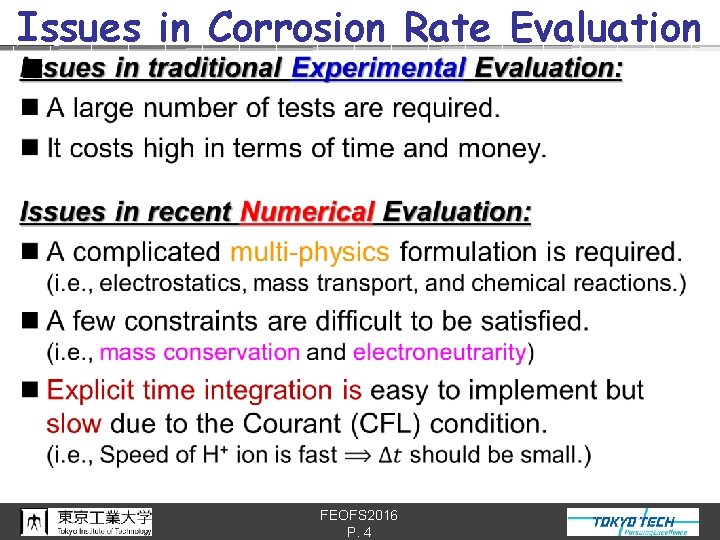
Issues in Corrosion Rate Evaluation n FEOFS 2016 P. 4
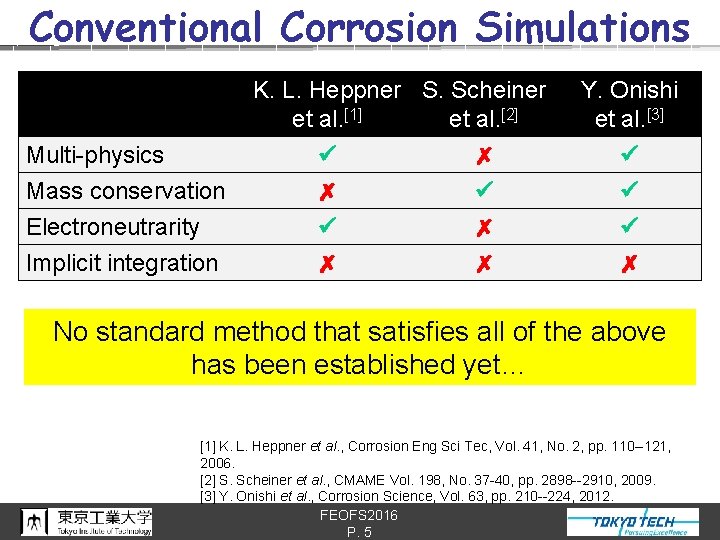
Conventional Corrosion Simulations Multi-physics Mass conservation Electroneutrarity Implicit integration K. L. Heppner S. Scheiner et al. [1] et al. [2] ✗ ✗ ✗ ✗ ✗ Y. Onishi et al. [3] ✗ No standard method that satisfies all of the above has been established yet… [1] K. L. Heppner et al. , Corrosion Eng Sci Tec, Vol. 41, No. 2, pp. 110 --121, 2006. [2] S. Scheiner et al. , CMAME Vol. 198, No. 37 -40, pp. 2898 --2910, 2009. [3] Y. Onishi et al. , Corrosion Science, Vol. 63, pp. 210 --224, 2012. FEOFS 2016 P. 5

Objective Developing a fast and accurate numerical multi-physics simulator for localized galvanic corrosion especially for CFRP/Al composite under a seawater film Table of Body Contents n Mechanism of galvanic corrosion on CFRP/Al composite under a seawater film n Our method to solve the corrosion problem n Validation of our method n Summary FEOFS 2016 P. 6
Mechanism of Galvanic Corrosion on CFRP/Al Composite under a Seawater Film FEOFS 2016 P. 7
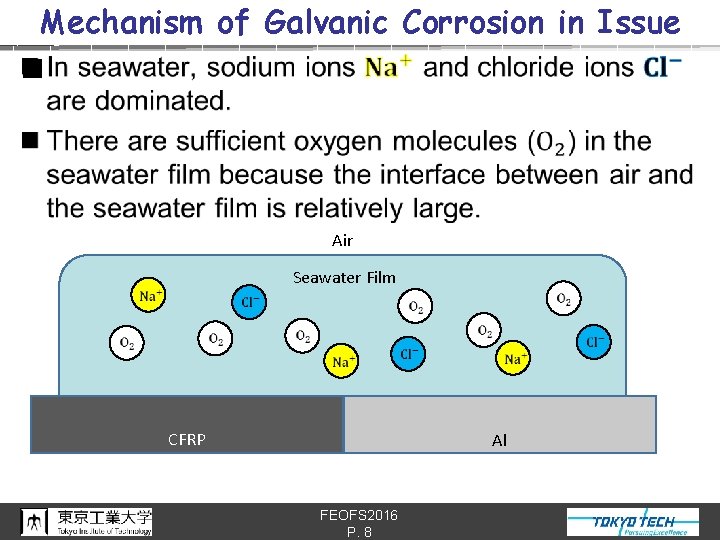
Mechanism of Galvanic Corrosion in Issue n Air Seawater Film CFRP Al FEOFS 2016 P. 8

Mechanism of Galvanic Corrosion in Issue n Air Seawater Film CFRP Flow of Current Higher Potential Al Lower Potential FEOFS 2016 P. 9
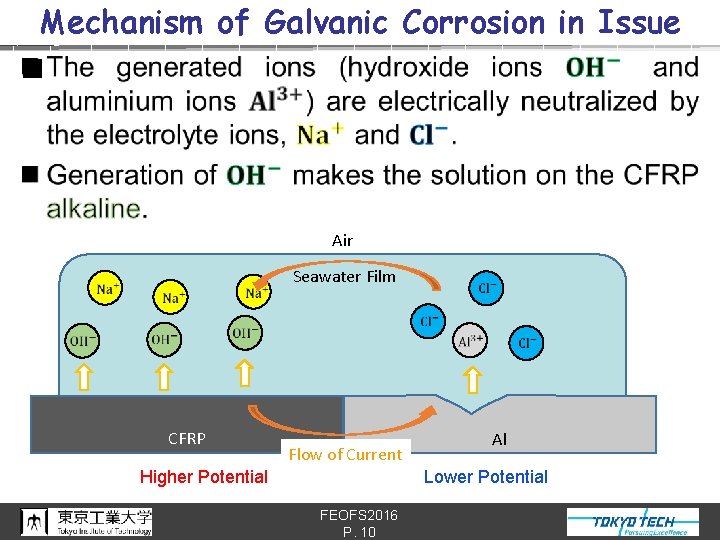
Mechanism of Galvanic Corrosion in Issue n Air Seawater Film CFRP Flow of Current Higher Potential Al Lower Potential FEOFS 2016 P. 10
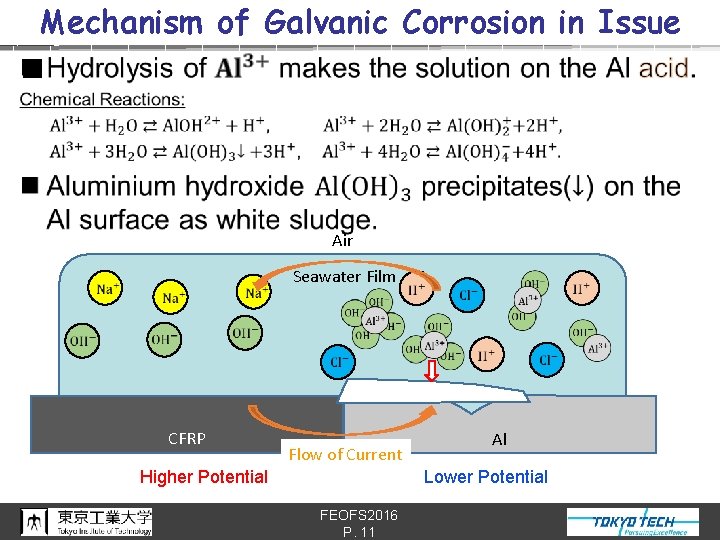
Mechanism of Galvanic Corrosion in Issue n Air Seawater Film CFRP Flow of Current Higher Potential Al Lower Potential FEOFS 2016 P. 11

Our Method to Solve the Corrosion Problem FEOFS 2016 P. 12
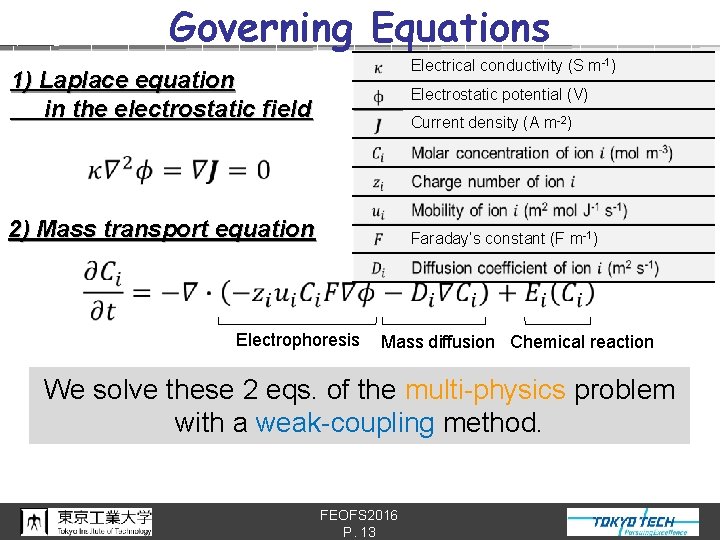
Governing Equations Electrical conductivity (S m-1) 1) Laplace equation in the electrostatic field Electrostatic potential (V) Current density (A m-2) 2) Mass transport equation Faraday’s constant (F m-1) Electrophoresis Mass diffusion Chemical reaction We solve these 2 eqs. of the multi-physics problem with a weak-coupling method. FEOFS 2016 P. 13
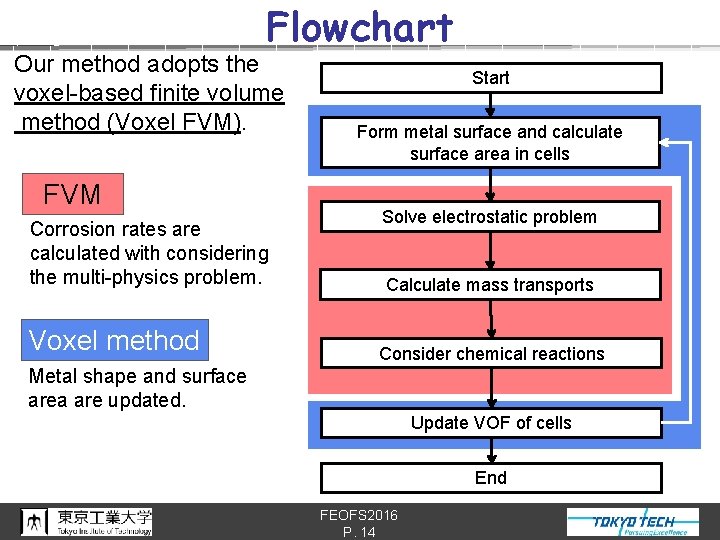
Flowchart Our method adopts the voxel-based finite volume method (Voxel FVM). FVM Corrosion rates are calculated with considering the multi-physics problem. Voxel method Start Form metal surface and calculate surface area in cells Solve electrostatic problem Calculate mass transports Consider chemical reactions Metal shape and surface area are updated. Update VOF of cells End FEOFS 2016 P. 14
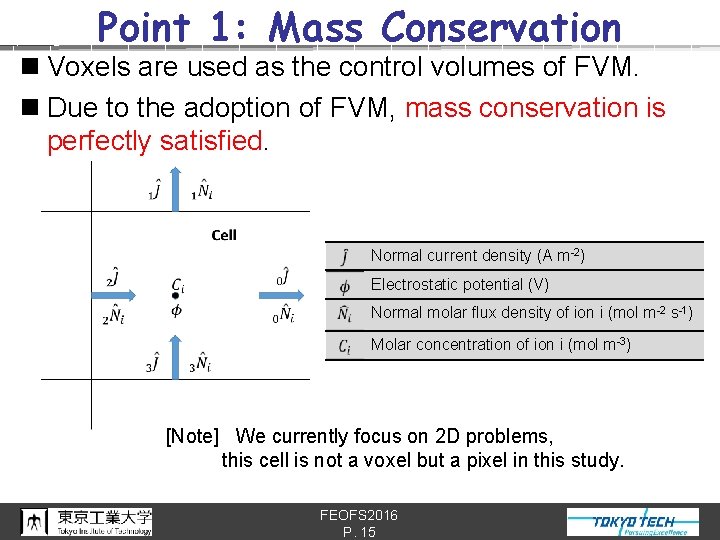
Point 1: Mass Conservation n Voxels are used as the control volumes of FVM. n Due to the adoption of FVM, mass conservation is perfectly satisfied. Normal current density (A m-2) Electrostatic potential (V) Normal molar flux density of ion i (mol m-2 s-1) Molar concentration of ion i (mol m-3) [Note] We currently focus on 2 D problems, this cell is not a voxel but a pixel in this study. FEOFS 2016 P. 15

n Point 2: Electroneutrality Normal current density (A m-2) Faraday’s constant (C mol-1) FEOFS 2016 P. 16
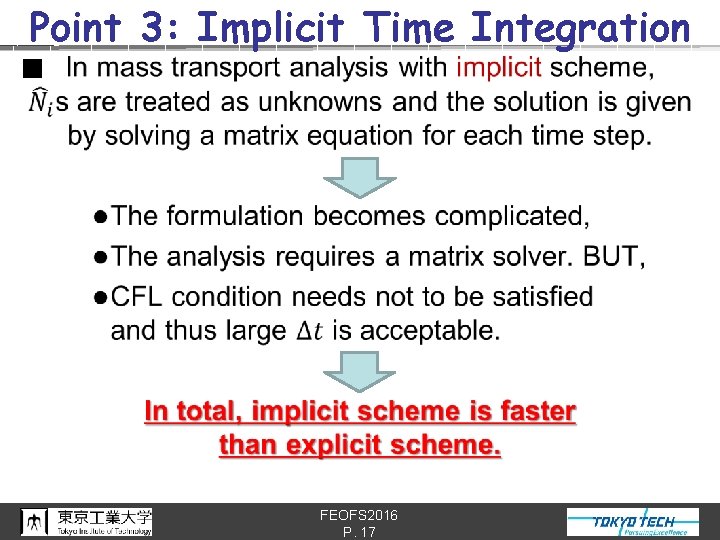
Point 3: Implicit Time Integration n FEOFS 2016 P. 17
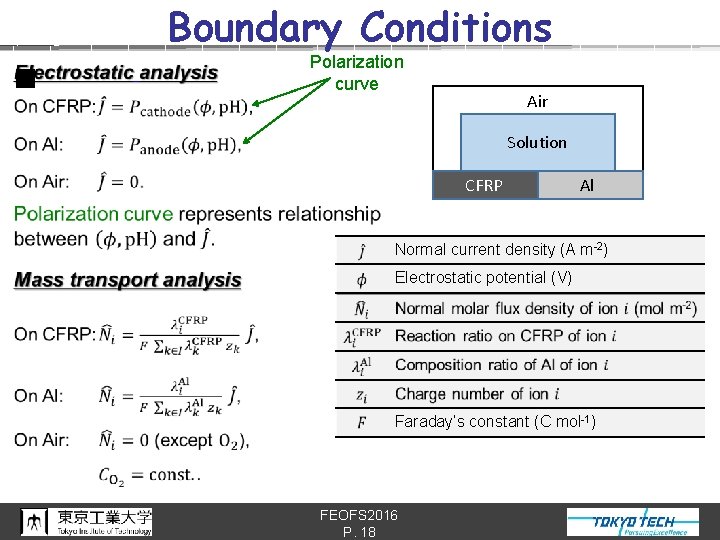
Boundary Conditions n Polarization curve Air Solution CFRP Al Normal current density (A m-2) Electrostatic potential (V) Faraday’s constant (C mol-1) FEOFS 2016 P. 18

Validation of Our Method FEOFS 2016 P. 19
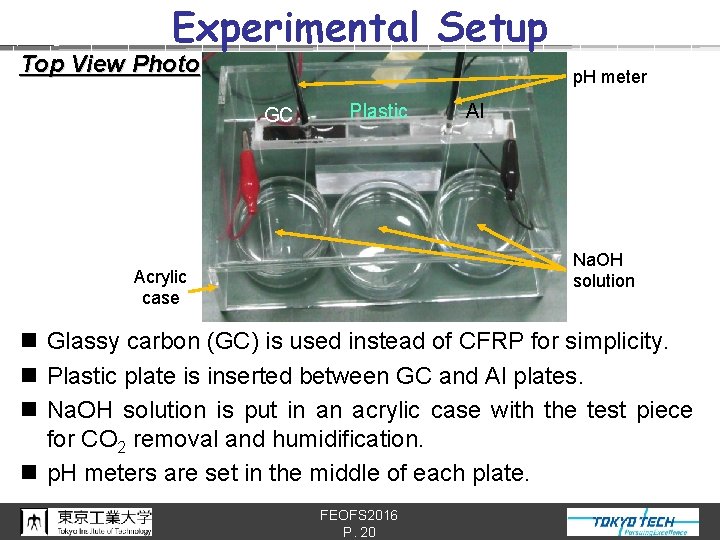
Experimental Setup Top View Photo p. H meter GC Plastic Al Na. OH solution Acrylic case n Glassy carbon (GC) is used instead of CFRP for simplicity. n Plastic plate is inserted between GC and Al plates. n Na. OH solution is put in an acrylic case with the test piece for CO 2 removal and humidification. n p. H meters are set in the middle of each plate. FEOFS 2016 P. 20
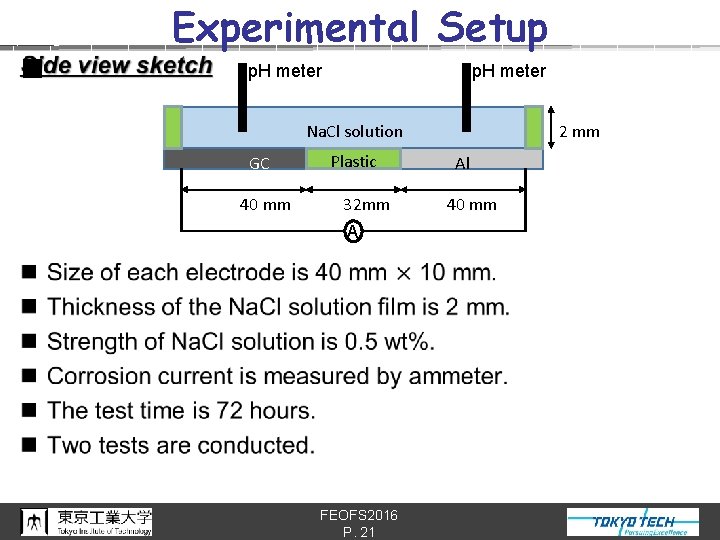
n Experimental Setup p. H meter 2 mm Na. Cl solution GC 40 mm Plastic 32 mm A FEOFS 2016 P. 21 Al 40 mm
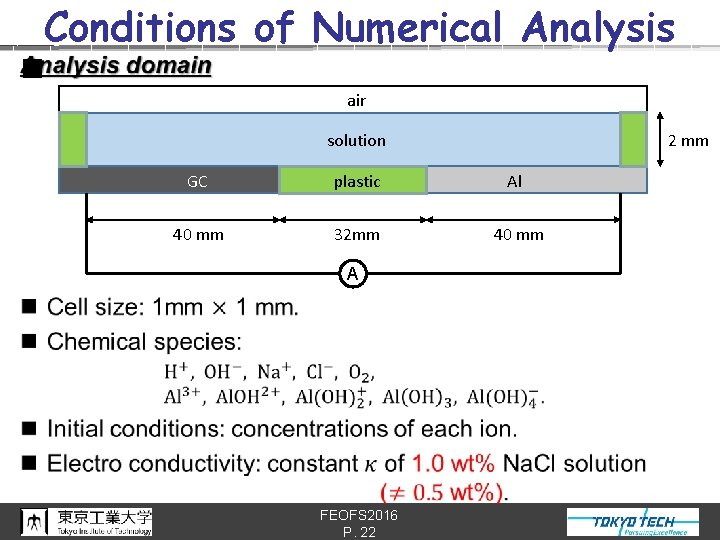
Conditions of Numerical Analysis n air 2 mm solution GC plastic Al 40 mm 32 mm 40 mm A FEOFS 2016 P. 22
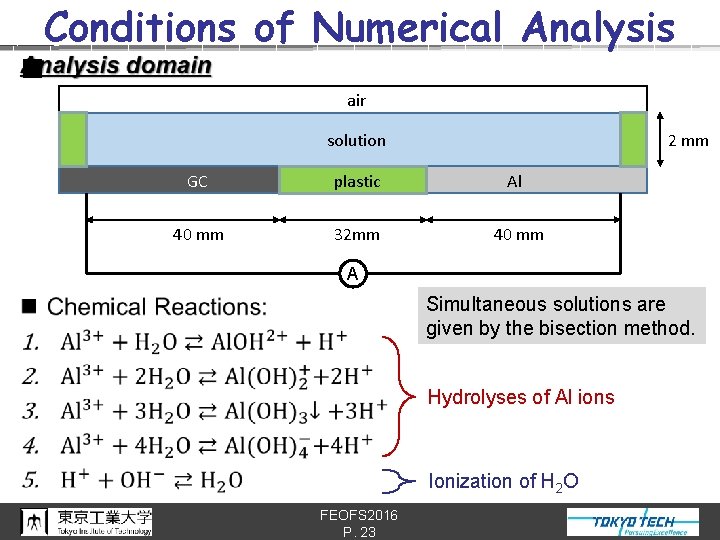
Conditions of Numerical Analysis n air 2 mm solution GC plastic Al 40 mm 32 mm 40 mm A Simultaneous solutions are given by the bisection method. Hydrolyses of Al ions Ionization of H 2 O FEOFS 2016 P. 23

Interval Photo Movie of Experiment FEOFS 2016 P. 24
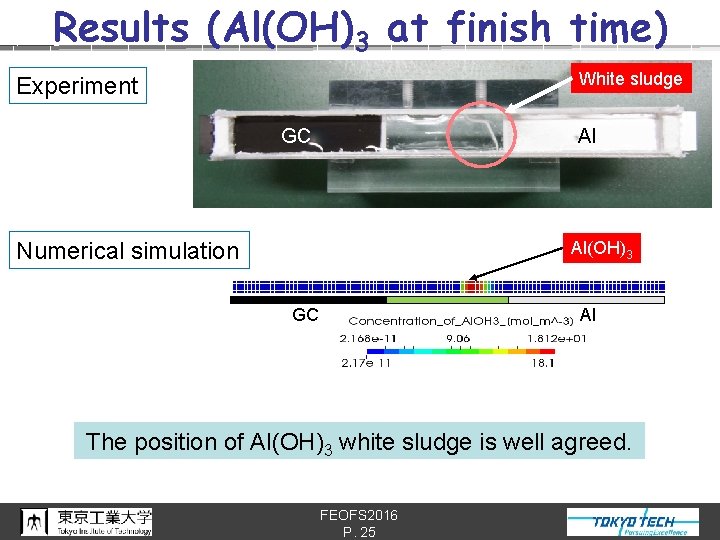
Results (Al(OH)3 at finish time) White sludge Experiment GC Al Numerical simulation Al(OH)3 p. H 13 GC p. H 3. 7 Al The position of Al(OH)3 white sludge is well agreed. FEOFS 2016 P. 25
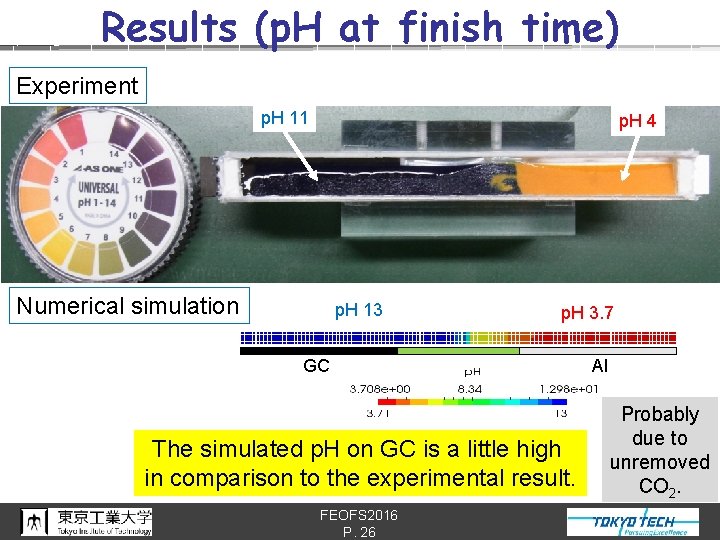
Results (p. H at finish time) Experiment p. H 11 p. H 4 Numerical simulation p. H 13 p. H 3. 7 GC The simulated p. H on GC is a little high in comparison to the experimental result. FEOFS 2016 P. 26 Al Probably due to unremoved CO 2.
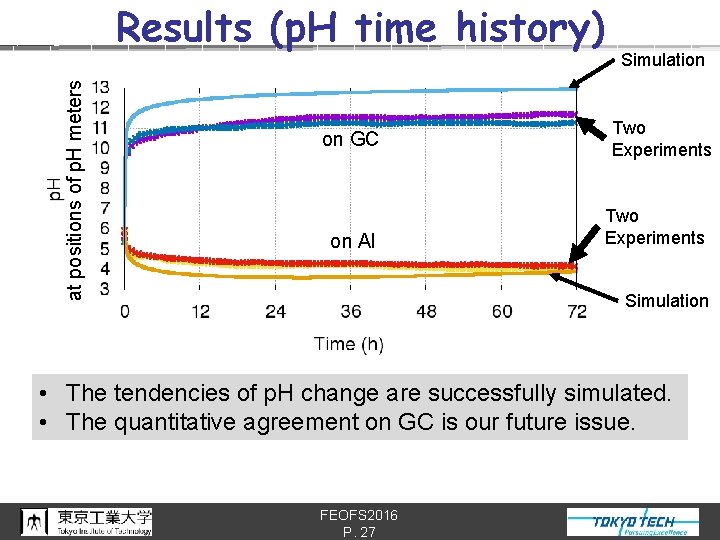
at positions of p. H meters Results (p. H time history) on GC on Al Simulation Two Experiments Simulation • The tendencies of p. H change are successfully simulated. • The quantitative agreement on GC is our future issue. FEOFS 2016 P. 27
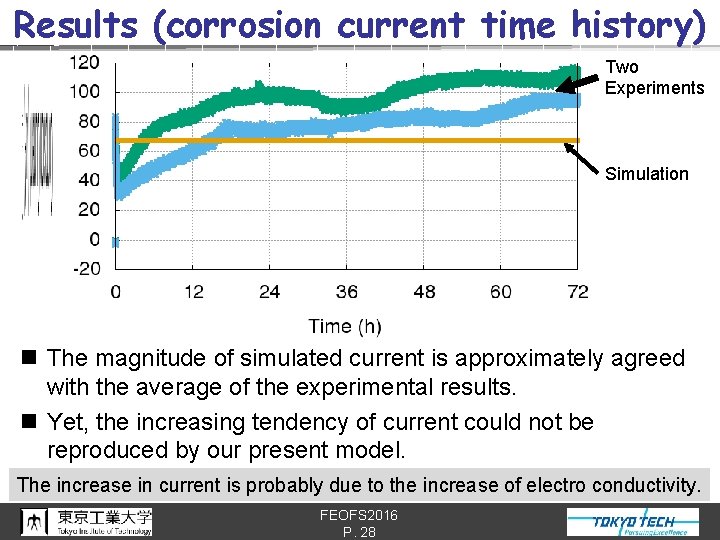
Results (corrosion current time history) Two Experiments Simulation n The magnitude of simulated current is approximately agreed with the average of the experimental results. n Yet, the increasing tendency of current could not be reproduced by our present model. The increase in current is probably due to the increase of electro conductivity. FEOFS 2016 P. 28
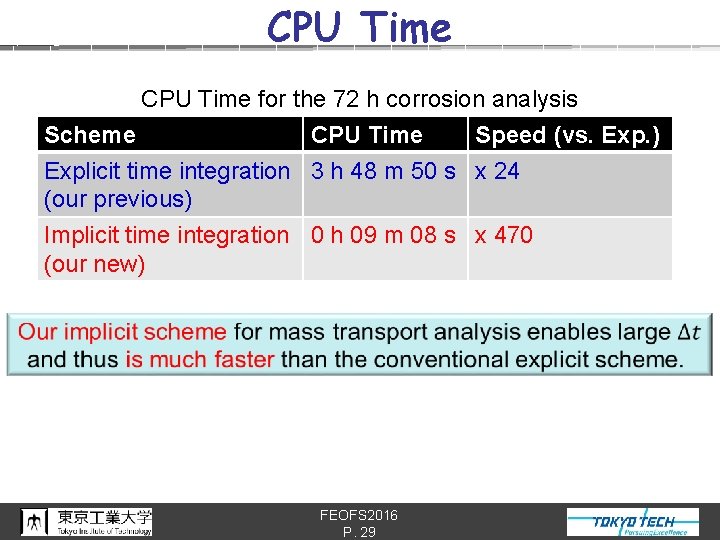
CPU Time for the 72 h corrosion analysis Scheme CPU Time Speed (vs. Exp. ) Explicit time integration 3 h 48 m 50 s x 24 (our previous) Implicit time integration 0 h 09 m 08 s x 470 (our new) FEOFS 2016 P. 29

Summary FEOFS 2016 P. 30
Summary n A multi-physics simulator for localized galvanic corrosion was developed. n It takes the followings into account. > electrophoresis > mass diffusion > chemical reactions > moving boundaries > mass conservation > electroneutrality > implicit time integration n A validation test with GC/Al composite revealed that our method was able to reproduce the distributions of p. H and white sludge, and corrosion current approximately. n A practical method for localized galvanic corrosion analysis is almost established. Thank you for your kind attention. FEOFS 2016 P. 31
- Slides: 31