Multimodal Imaging MEGNIRS integration Neurovascular coupling Nearinfrared spectroscopy



- Slides: 33

Multimodal Imaging: MEG-NIRS integration Neuro-vascular coupling Near-infrared spectroscopy Magnetoencephalography and DC-magnetoencephalography Signal processing: Independent component analysis Results Summary T. H. Sander, M. Moeller, S. Leistner, A. Liebert, H. Wabnitz, M. Burghoff, G. Curio, B. M. Mackert, R. Macdonald, L. Trahms Physikalisch. Technische Bundesanstalt

Neuro-vascular coupling

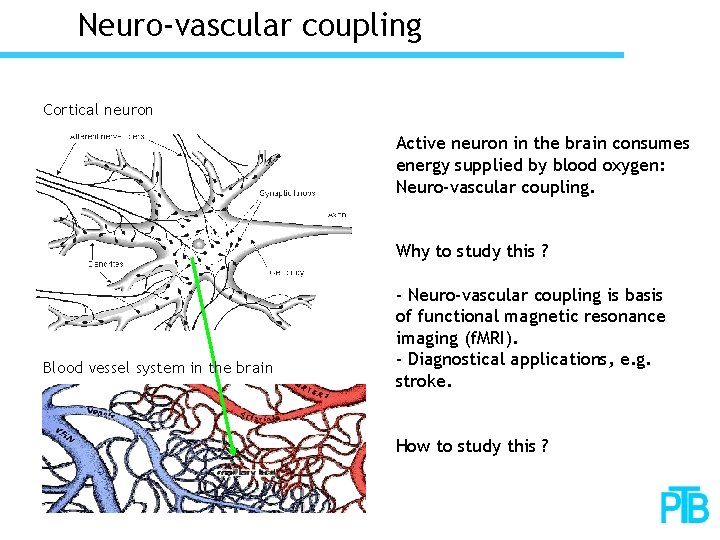
Neuro-vascular coupling Cortical neuron Active neuron in the brain consumes energy supplied by blood oxygen: Neuro-vascular coupling. Why to study this ? Blood vessel system in the brain - Neuro-vascular coupling is basis of functional magnetic resonance imaging (f. MRI). - Diagnostical applications, e. g. stroke. How to study this ?

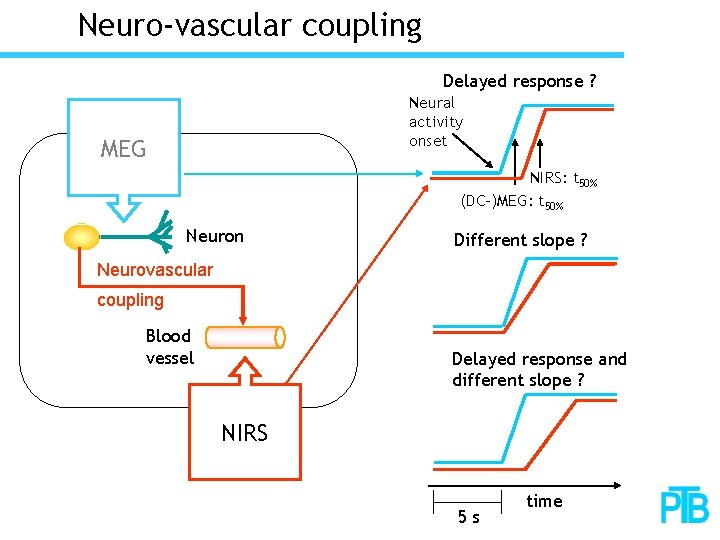
Neuro-vascular coupling Delayed response ? Neural activity onset MEG NIRS: t 50% (DC-)MEG: t 50% Neuron Different slope ? Neurovascular coupling Blood vessel Delayed response and different slope ? NIRS 5 s time

Near-infrared spectroscopy (NIRS)

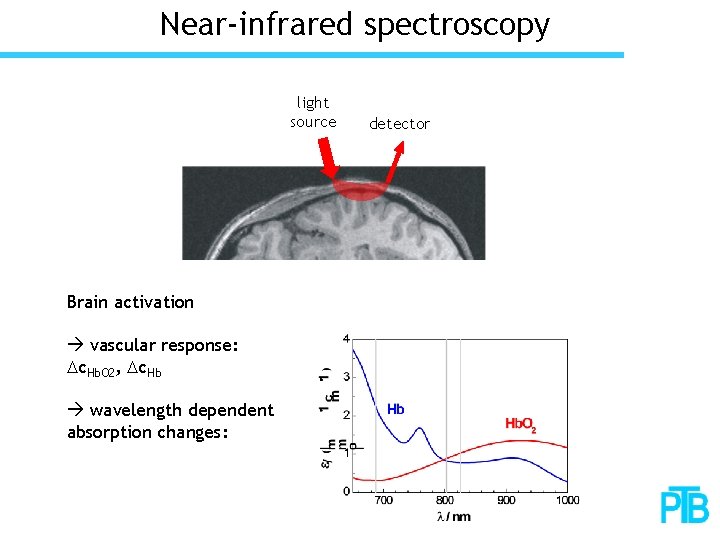
Near-infrared spectroscopy light source Brain activation vascular response: Dc. Hb. O 2, Dc. Hb wavelength dependent absorption changes: detector

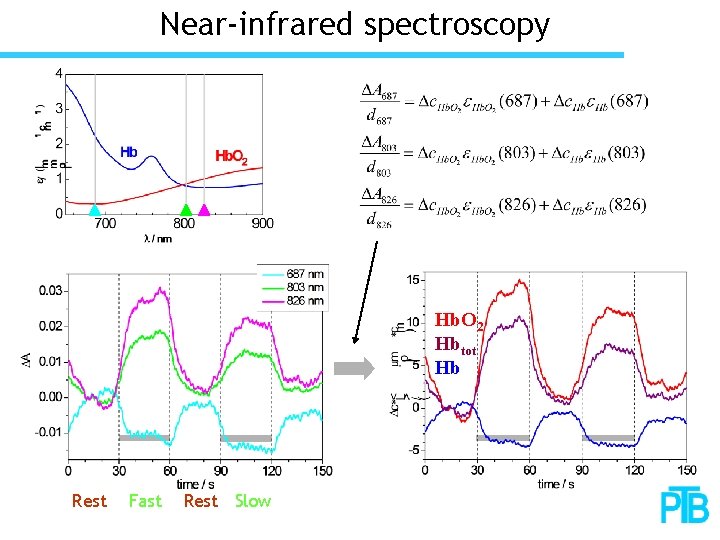
Near-infrared spectroscopy Hb. O 2 Hbtot Hb Rest Fast Rest Slow

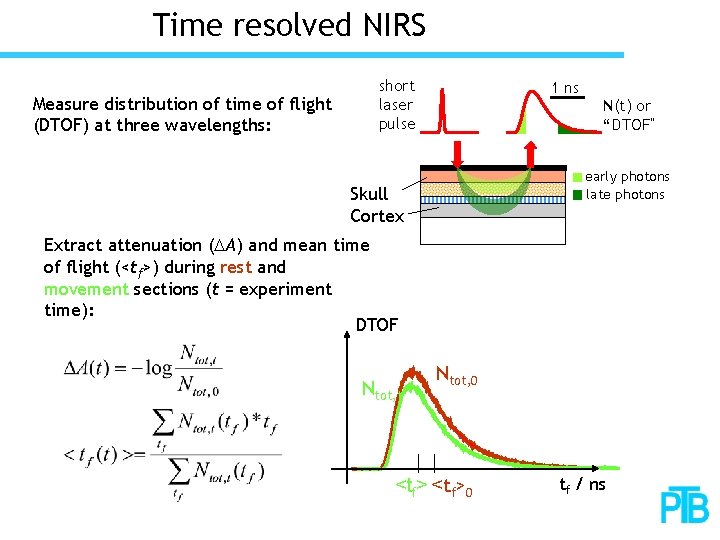
Time resolved NIRS Measure distribution of time of flight (DTOF) at three wavelengths: short laser pulse 1 ns N(t) or “DTOF” early photons late photons Skull Cortex Extract attenuation (DA) and mean time of flight (<tf>) during rest and movement sections (t = experiment time): DTOF Ntot, t Ntot, 0 <tf>0 tf / ns

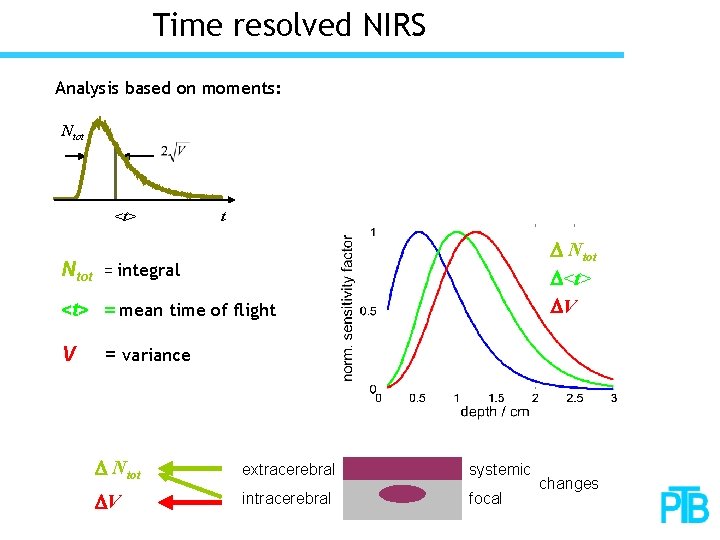
Time resolved NIRS Analysis based on moments: Ntot <t> Ntot t D Ntot D<t> DV = integral <t> = mean time of flight V = variance D Ntot extracerebral systemic DV intracerebral focal changes

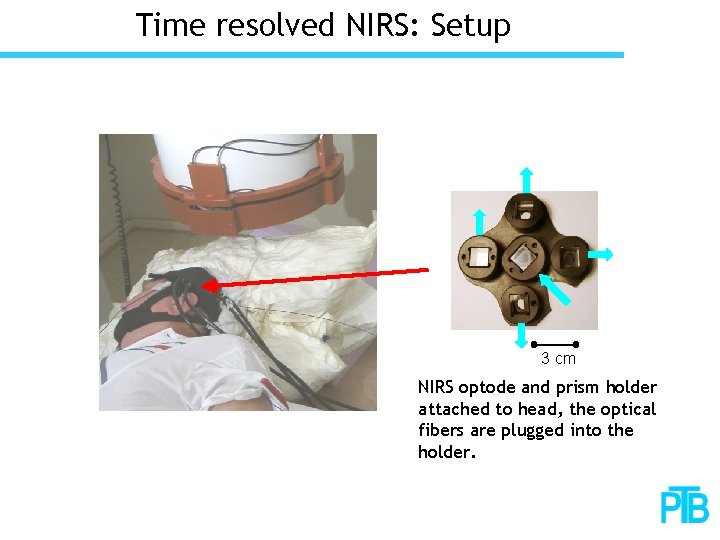
Time resolved NIRS: Setup 3 cm NIRS optode and prism holder attached to head, the optical fibers are plugged into the holder.

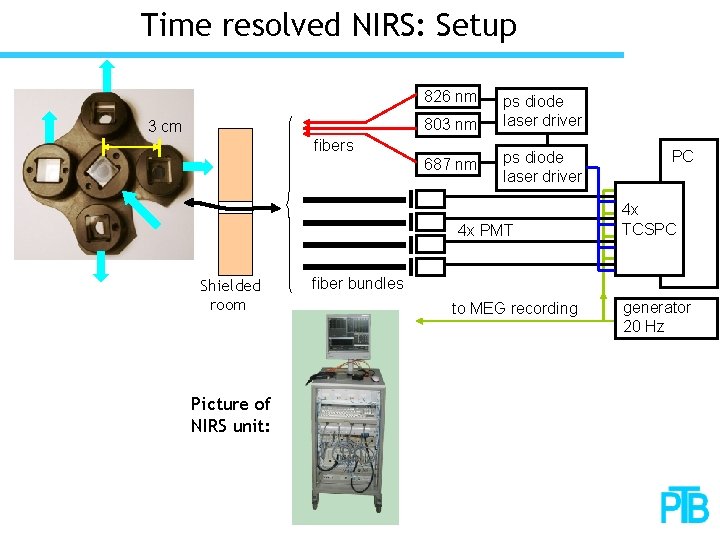
Time resolved NIRS: Setup 826 nm 803 nm 3 cm fibers 687 nm ps diode laser driver 4 x PMT Shielded room Picture of NIRS unit: PC 4 x TCSPC fiber bundles to MEG recording generator 20 Hz

Magnetoencephalography and DC-MEG

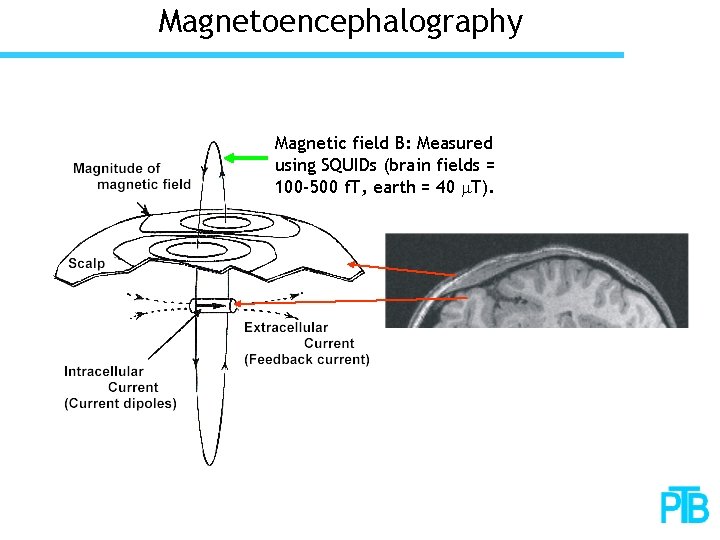
Magnetoencephalography Magnetic field B: Measured using SQUIDs (brain fields = 100 -500 f. T, earth = 40 m. T).

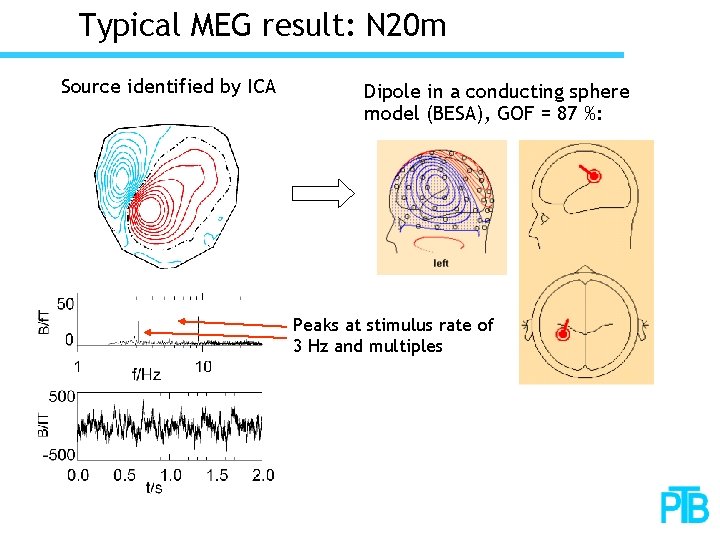
Typical MEG result: N 20 m Source identified by ICA Dipole in a conducting sphere model (BESA), GOF = 87 %: Peaks at stimulus rate of 3 Hz and multiples

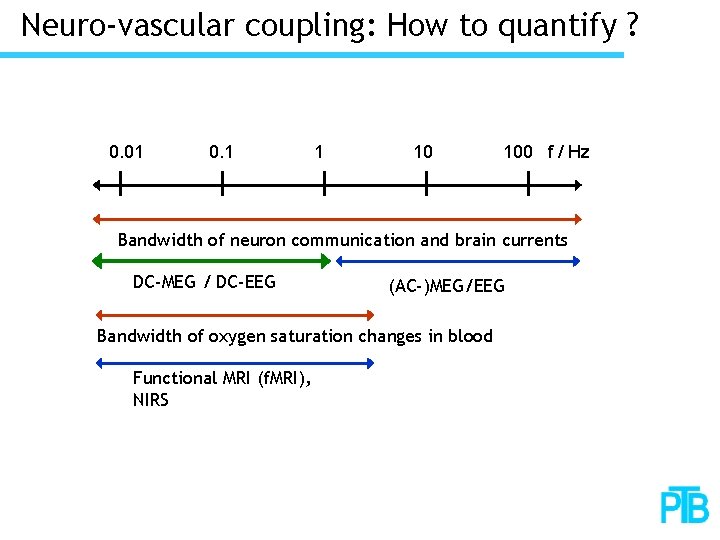
Neuro-vascular coupling: How to quantify ? 0. 01 0. 1 1 10 100 f / Hz Bandwidth of neuron communication and brain currents DC-MEG / DC-EEG (AC-)MEG/EEG Bandwidth of oxygen saturation changes in blood Functional MRI (f. MRI), NIRS

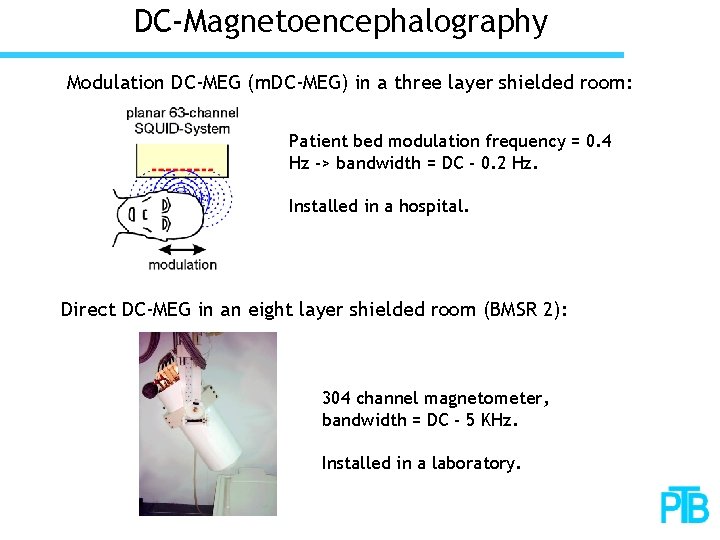
DC-Magnetoencephalography Modulation DC-MEG (m. DC-MEG) in a three layer shielded room: Patient bed modulation frequency = 0. 4 Hz -> bandwidth = DC - 0. 2 Hz. Installed in a hospital. Direct DC-MEG in an eight layer shielded room (BMSR 2): 304 channel magnetometer, bandwidth = DC - 5 KHz. Installed in a laboratory.

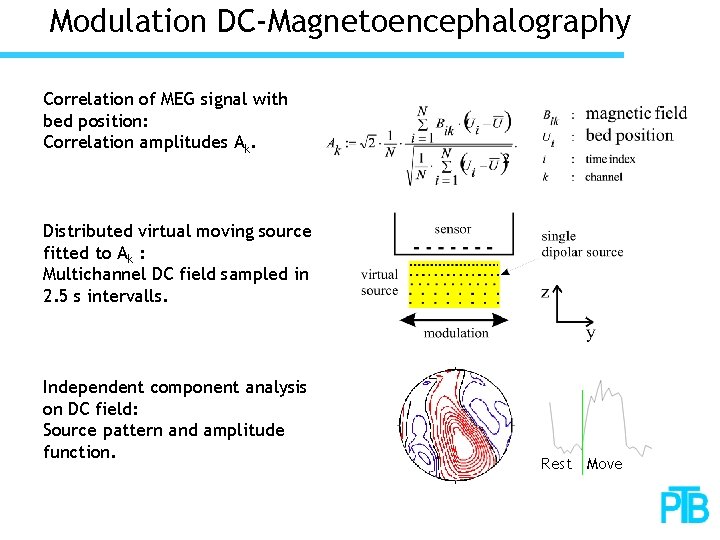
Modulation DC-Magnetoencephalography Correlation of MEG signal with bed position: Correlation amplitudes Ak. Distributed virtual moving source fitted to Ak : Multichannel DC field sampled in 2. 5 s intervalls. Independent component analysis on DC field: Source pattern and amplitude function. Rest Move

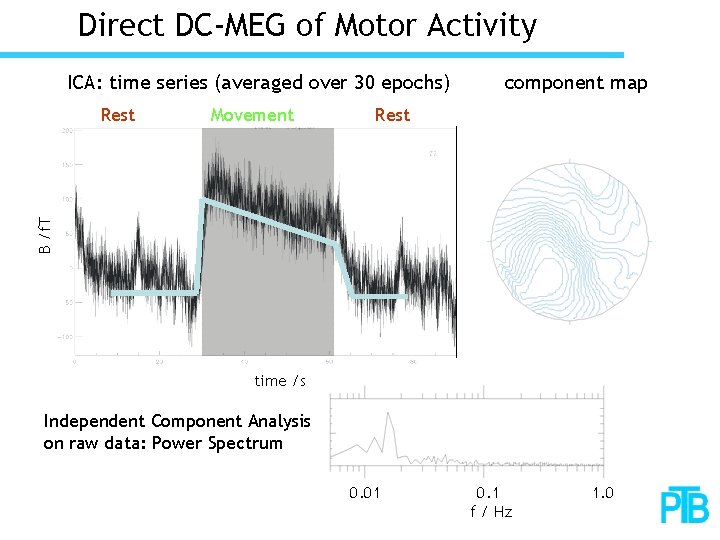
Direct DC-MEG of Motor Activity ICA: time series (averaged over 30 epochs) Movement Rest B /f. T Rest component map time /s Independent Component Analysis on raw data: Power Spectrum 0. 01 0. 1 f / Hz 1. 0

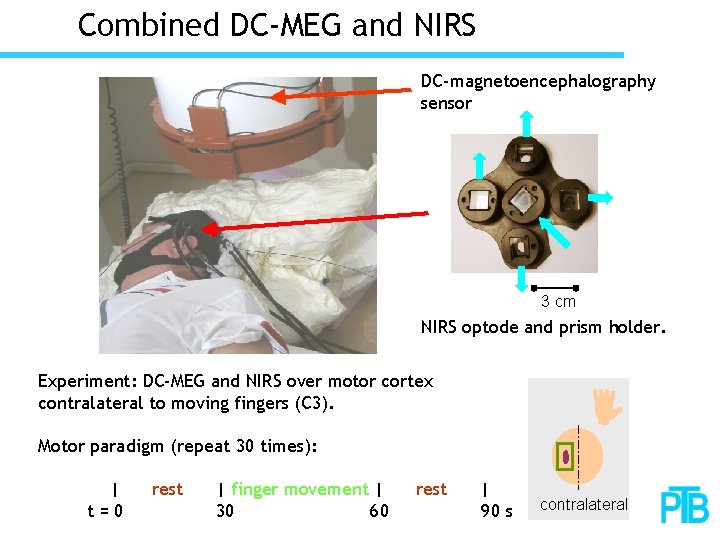
Combined DC-MEG and NIRS DC-magnetoencephalography sensor 3 cm NIRS optode and prism holder. Experiment: DC-MEG and NIRS over motor cortex contralateral to moving fingers (C 3). Motor paradigm (repeat 30 times): | t=0 rest | finger movement | 30 60 rest | 90 s contralateral

Signal processing: Independent Component Analysis

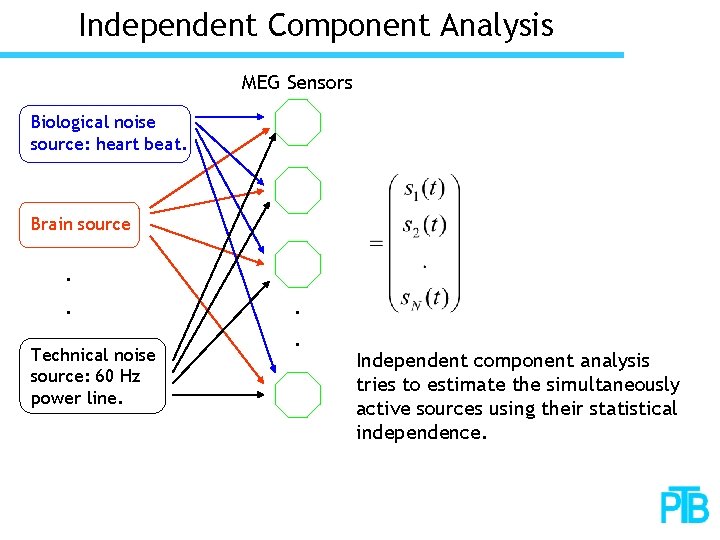
Independent Component Analysis MEG Sensors Biological noise source: heart beat. Brain source. . Technical noise source: 60 Hz power line. . . Independent component analysis tries to estimate the simultaneously active sources using their statistical independence.

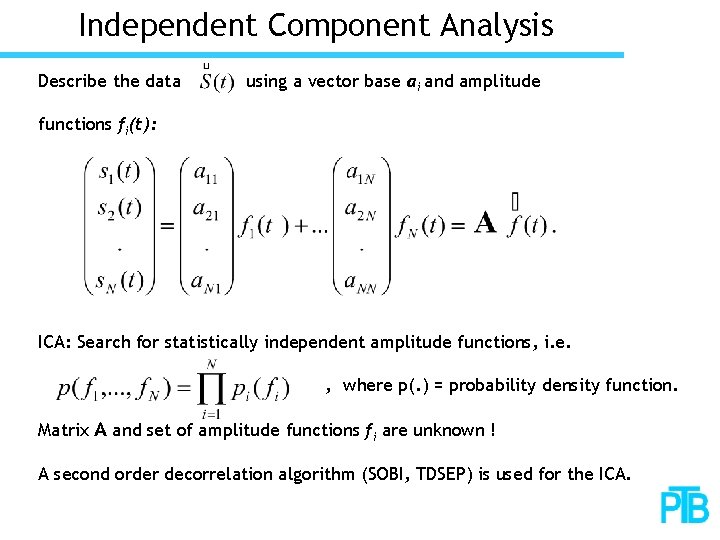
Independent Component Analysis Describe the data using a vector base ai and amplitude functions fi(t): ICA: Search for statistically independent amplitude functions, i. e. , where p(. ) = probability density function. Matrix A and set of amplitude functions fi are unknown ! A second order decorrelation algorithm (SOBI, TDSEP) is used for the ICA.

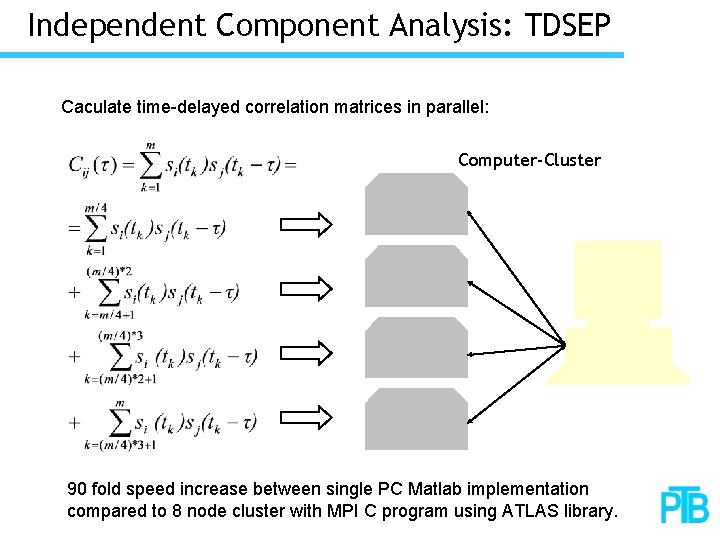
Independent Component Analysis: TDSEP Caculate time-delayed correlation matrices in parallel: Computer-Cluster 90 fold speed increase between single PC Matlab implementation compared to 8 node cluster with MPI C program using ATLAS library.

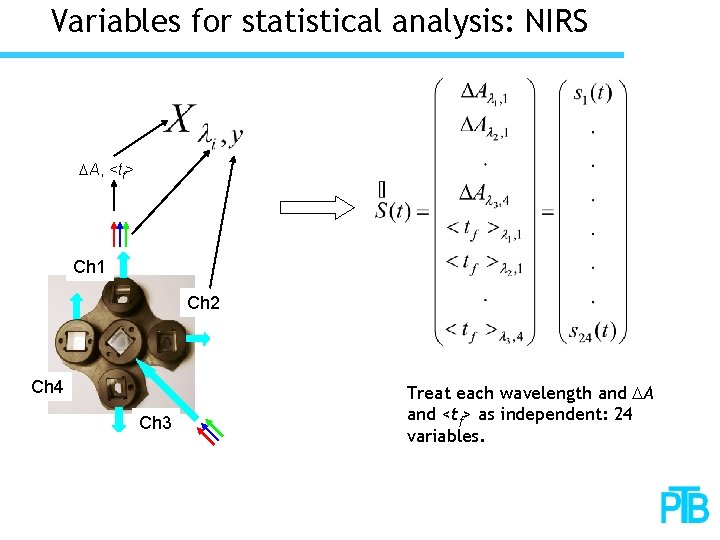
Variables for statistical analysis: NIRS DA, <tf> Ch 1 Ch 2 Ch 4 Ch 3 Treat each wavelength and DA and <tf> as independent: 24 variables.

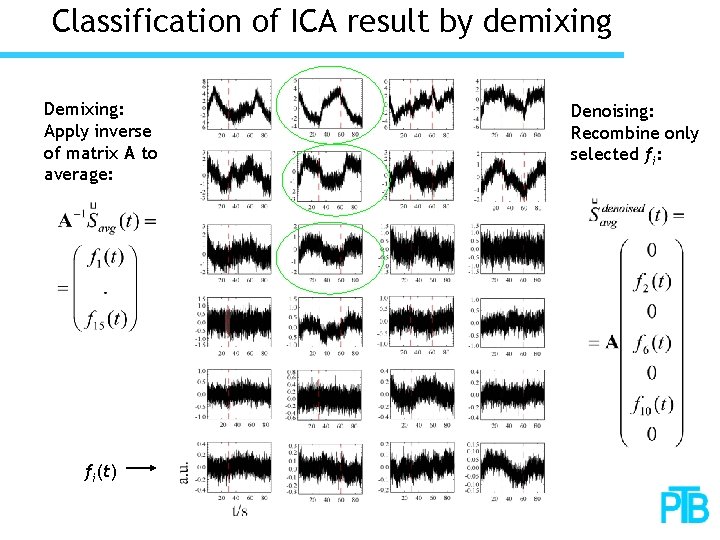
Classification of ICA result by demixing Demixing: Apply inverse of matrix A to average: fi(t) Denoising: Recombine only selected fi:

Results: Group study on normal subjects (NIRS and m. DC-MEG) First results for unmodulated DC-MEG combined with NIRS Feasibility study with subacute stroke patients (NIRS and m. DC-MEG)

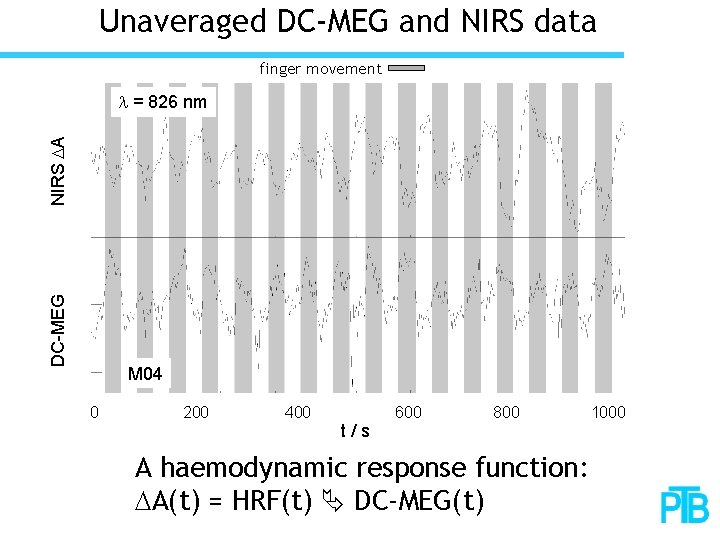
Unaveraged DC-MEG and NIRS data finger movement DC-MEG NIRS DA l = 826 nm M 04 0 200 400 t/s 600 800 A haemodynamic response function: DA(t) = HRF(t) DC-MEG(t) 1000

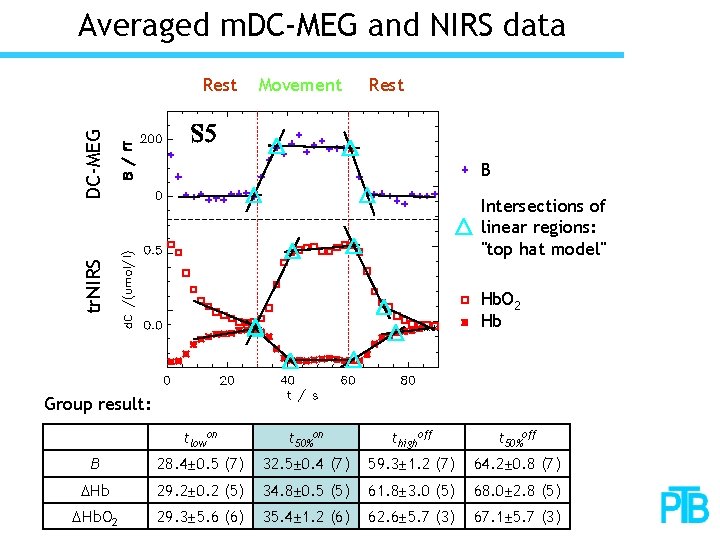
Averaged m. DC-MEG and NIRS data Movement Rest DC-MEG Rest B tr. NIRS Intersections of linear regions: "top hat model" Hb. O 2 Hb Group result: tlowon t 50%on thighoff t 50%off B 28. 4± 0. 5 (7) 32. 5± 0. 4 (7) 59. 3± 1. 2 (7) 64. 2± 0. 8 (7) DHb 29. 2± 0. 2 (5) 34. 8± 0. 5 (5) 61. 8± 3. 0 (5) 68. 0± 2. 8 (5) DHb. O 2 29. 3± 5. 6 (6) 35. 4± 1. 2 (6) 62. 6± 5. 7 (3) 67. 1± 5. 7 (3)

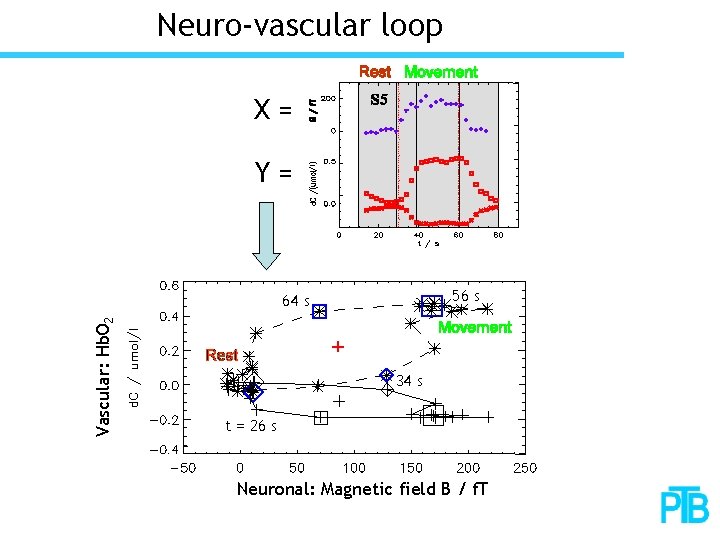
Neuro-vascular loop X= Y= 56 s Vascular: Hb. O 2 64 s 34 s t = 26 s Neuronal: Magnetic field B / f. T

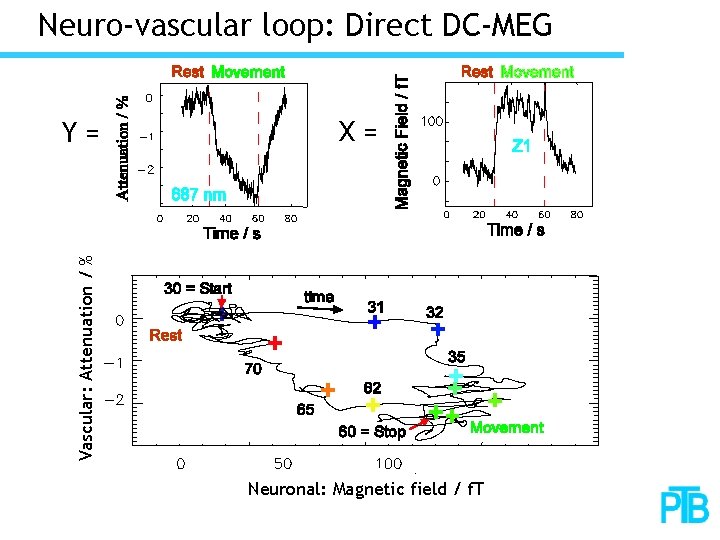
Neuro-vascular loop: Direct DC-MEG X= Vascular: Attenuation / % Y= Neuronal: Magnetic field / f. T

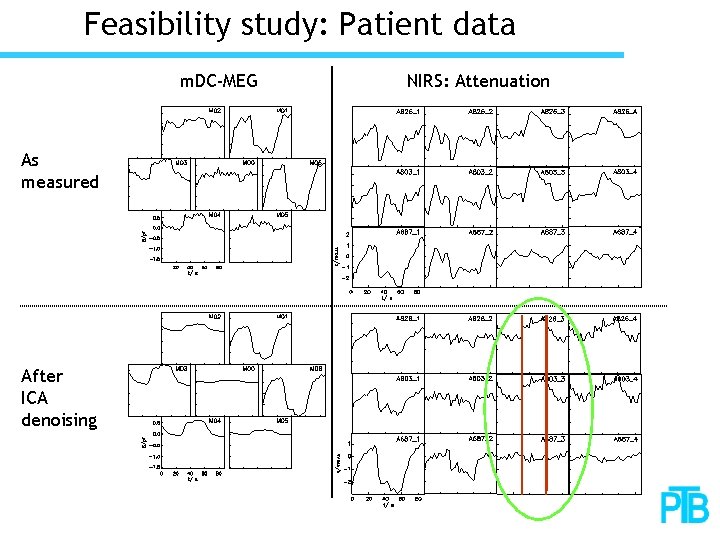
Feasibility study: Patient data m. DC-MEG As measured After ICA denoising NIRS: Attenuation

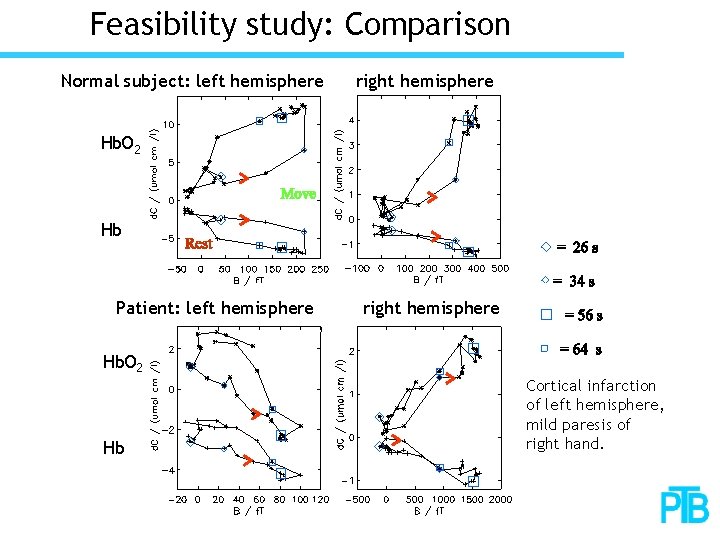
Feasibility study: Comparison Normal subject: left hemisphere right hemisphere Hb. O 2 Hb Patient: left hemisphere right hemisphere Hb. O 2 Hb Cortical infarction of left hemisphere, mild paresis of right hand.

Summary Combined DC-MEG and time resolved NIRS technique demonstrated. Independent Component Anaylysis essential for signal conditioning. Neuro-vascular coupling characterised for group of healthy subjects. Neuro-vascular loop introduced. Outlook: NIRS imager with 9 sources and 4 detectors Estimate haemodynamic response function with high temporal resolution Multi-modal independent component analysis combining NIRS and DC-MEG Supported by Berlin Neuroimaging Center