Multidimensional Parallel Column Gas Chromatography P M Owens













- Slides: 23

Multidimensional Parallel Column Gas Chromatography P. M. Owens and D. W. Loehle Center for Molecular Sciences United States Military Academy West Point, NY 10996


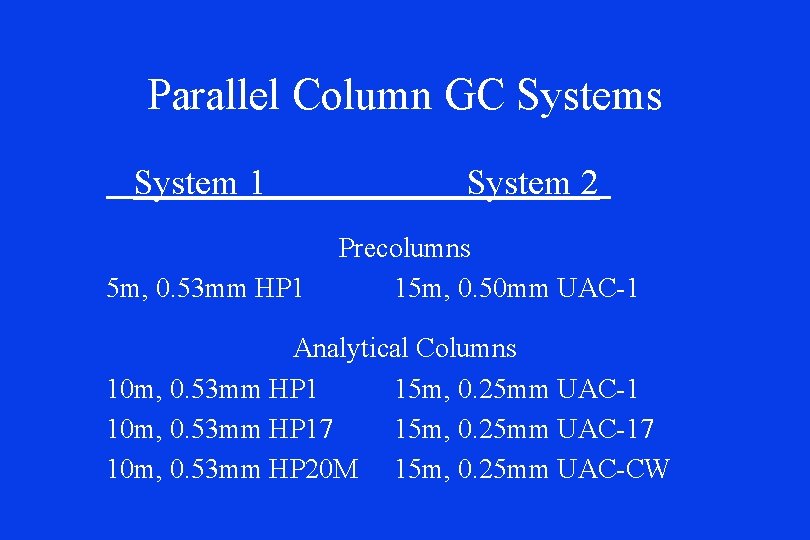
Parallel Column GC Systems System 1 5 m, 0. 53 mm HP 1 System 2 Precolumns 15 m, 0. 50 mm UAC-1 Analytical Columns 10 m, 0. 53 mm HP 1 15 m, 0. 25 mm UAC-1 10 m, 0. 53 mm HP 17 15 m, 0. 25 mm UAC-17 10 m, 0. 53 mm HP 20 M 15 m, 0. 25 mm UAC-CW

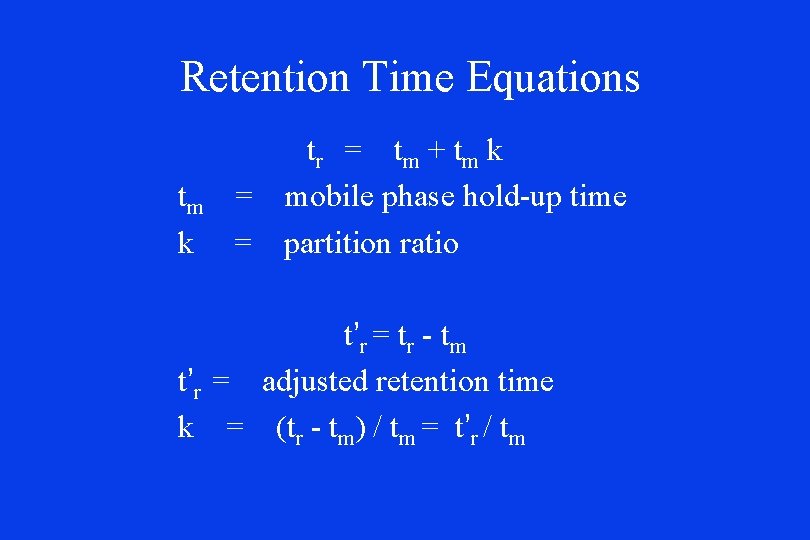
Retention Time Equations tm k tr = t m + t m k = mobile phase hold-up time = partition ratio t’r = tr - tm t’r = adjusted retention time k = (tr - tm) / tm = t’r / tm

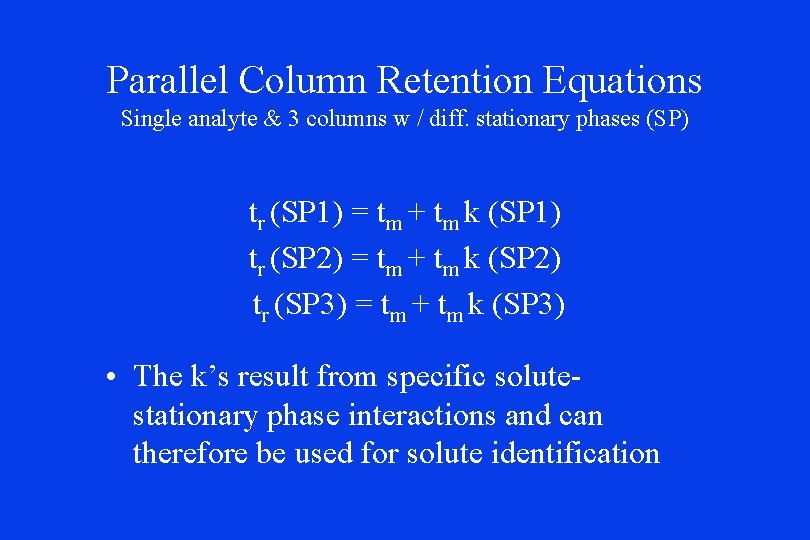
Parallel Column Retention Equations Single analyte & 3 columns w / diff. stationary phases (SP) tr (SP 1) = tm + tm k (SP 1) tr (SP 2) = tm + tm k (SP 2) tr (SP 3) = tm + tm k (SP 3) • The k’s result from specific solutestationary phase interactions and can therefore be used for solute identification

Retention Time Calibration Day 1 t. IS (1) t. AN (1) = = tm 1 + tm 1 k. IS tm 1 + tm 1 k. AN Day 2 t. IS (2) t. AN (2) = = tm 2 + F * tm 2 k. IS tm 2 + F * tm 2 k. AN F corrects for changes in k

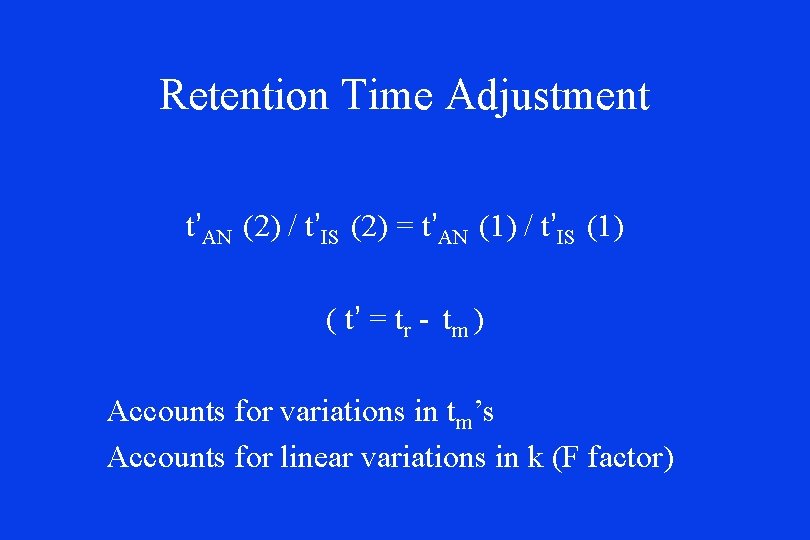
Retention Time Adjustment t’AN (2) / t’IS (2) = t’AN (1) / t’IS (1) ( t’ = tr - tm ) Accounts for variations in tm’s Accounts for linear variations in k (F factor)

Relative Retention (a) Libraries a A, IS = t’A (2) / t’IS(2) = t’A (1) / t’IS (1) • Generate GC library to tabulate a’s for each compound on all stationary phases • Run int. std. with all analyte & library runs • Since a’s are T-dependent, run all samples with identical temperature programs

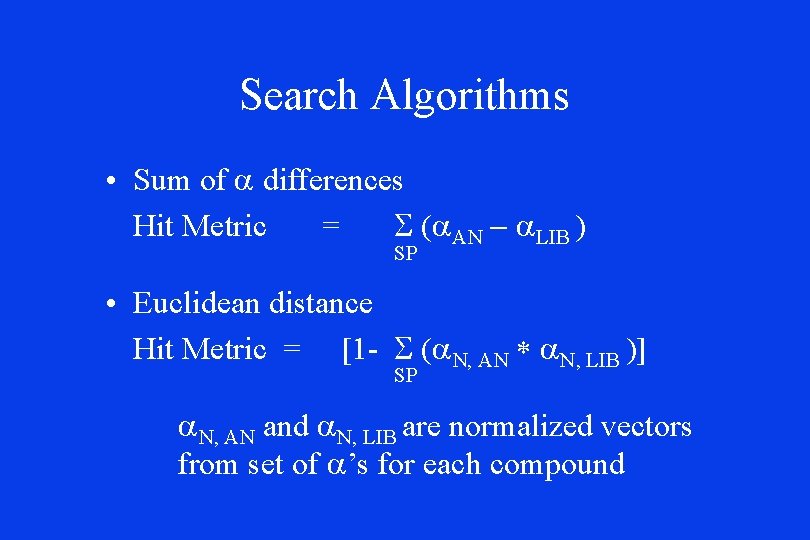
Search Algorithms • Sum of a differences Hit Metric = S (a. AN - a. LIB ) SP • Euclidean distance Hit Metric = [1 - S (a. N, AN * a. N, LIB )] SP a. N, AN and a. N, LIB are normalized vectors from set of a’s for each compound

Search Results HP 20 M • Sum of a differences Cyclooctane 1. 85 Cyclooctadiene 1. 93 1 -Heptanol 1. 84 • Euclidean Search Cyclooctane 1. 85 Nonanoic Acid 3. 05 Octanoic Acid 2. 71 HP 17 HP 1 1. 77 1. 93 1. 84 1. 46 1. 49 1. 58 1. 77 2. 87 2. 57 1. 46 2. 19

Chromatography Relations KD k b = = = KD = k b Distribution constant Partition ratio Phase ratio (Vg / Vs ) KD depends on three variables: 1) temperature, 2) solute, & 3) stationary phase

Retention & Thermodynamics KD = k b tr = t m + t m k DG = -RT ln KD DG = DH - T DS ln k + ln b = -DH / R T + DS / R ln k = - DH / R (1 / T) + DS / R - ln b



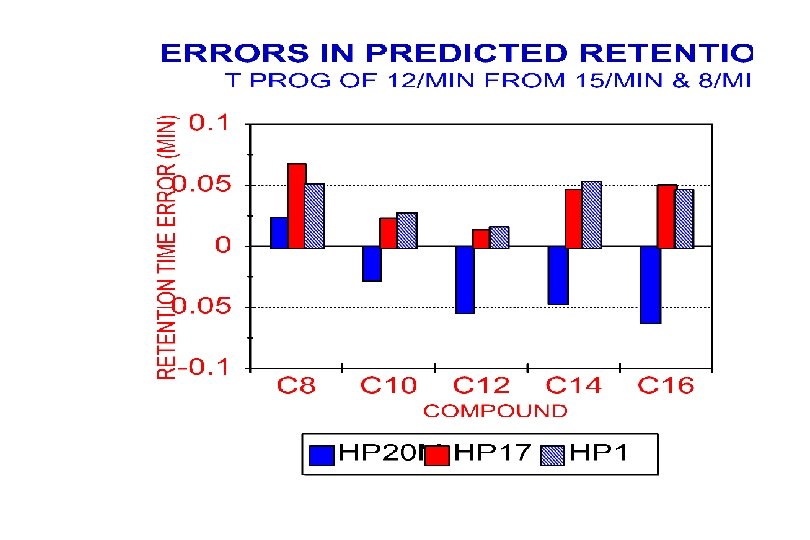
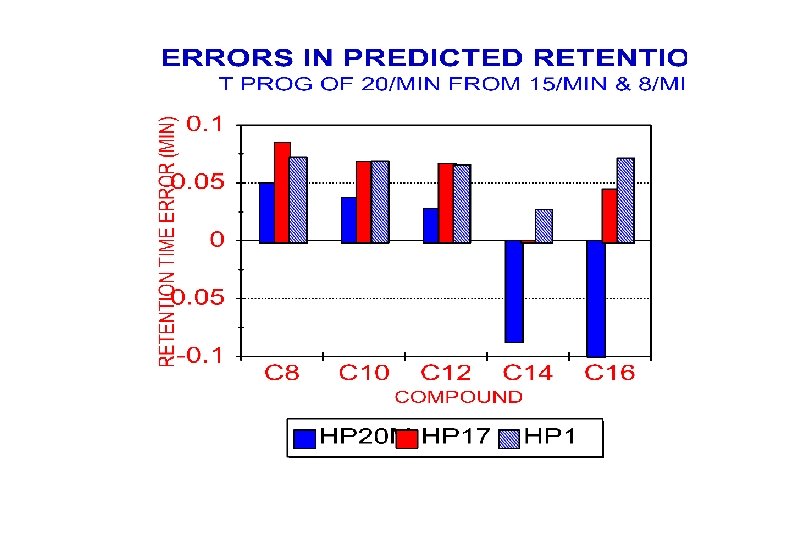
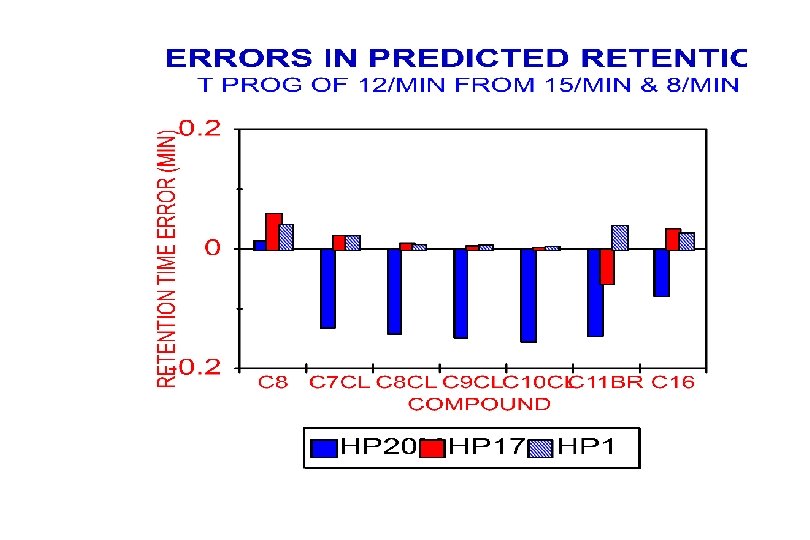
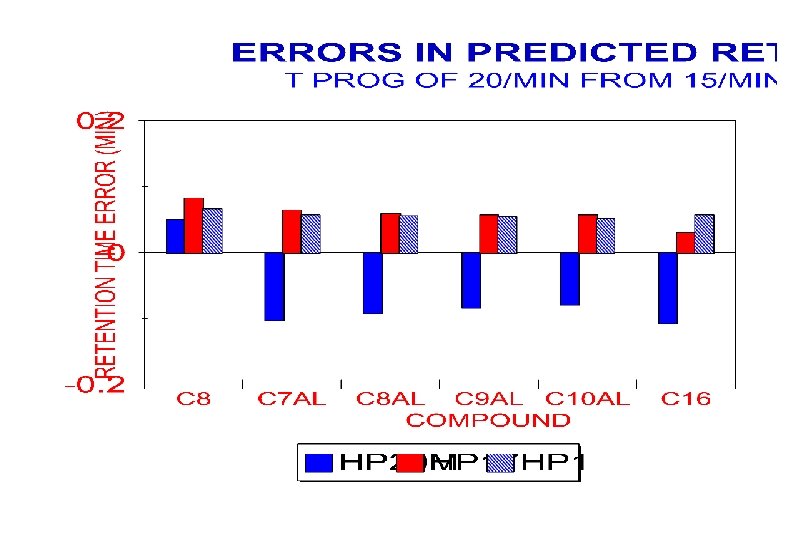
Retention Prediction Errors (CV) Prediction Used T-progs of 8 o. C/min & 15 o. C/min Sample (C 7 -C 11) 12 C/min 20 C/min Alkanes (C 8 -C 16) Halogenated Ketones Aldehydes Alkanes (C 9 -C 15) 0. 27% 0. 38% 0. 56% 0. 61% 0. 33% 0. 51% 0. 87% 0. 59% 0. 62% 0. 45%





Predict Retention for Analyte GC conditions Library Compounds Predict Analyte Retention under Lib. GC Conditions X Single Analyte Chromatogram

Parallel Column Gas Chromatography • Measures interaction on multiple stationary phases - a separate dimension of analyte information • Requires the use of internal standards to characterize GC operating conditions • Thermodynamic modeling allows adjustment of library retentions to current operating conditions

Future Areas of Focus • Interinstrument variability assessment • Development of calibration procedures to minimize retention prediction errors • Optimization of stationary phase selection • Evaluation of an increased number of parallel columns • Application for complex mixture analysis

Acknowledgements • Association of Graduates and Army Research Office • Beverly S. Scott & Rodney S. Gonzalez • Tony Weaver • Department of Chemistry, USMA