Multicolor Flow Cytometry Optimizing Performance for Expanding Colors













- Slides: 98

Multicolor Flow Cytometry: Optimizing Performance for Expanding Colors into High Parameter DATA collection Anthony L. Steichen, Ph. D. High Parameter Field Application Scientist For Research Use Only. Not for use in diagnostic or therapeutic procedures. APC-Cy 7: US Patent 5, 714, 386 Alexa Fluor® is a registered trademark and Pacific Blue™ is a trademark of Life Technologies Corporation. CF is a trademark of Biotium, Inc. Cy™ is a trademark of GE Healthcare. Cy™ dyes are subject to proprietary rights of GE Healthcare and Carnegie Mellon University and are made and sold under license from GE Healthcare only for research and in vitro diagnostic use. Any other use requires a commercial sublicense from GE Healthcare, 800 Centennial Avenue, Piscataway, NJ 08855 -1327, USA. BD, BD Logo and all other trademarks are property of Becton, Dickinson and Company. © 2014 BD bdbiosciences. com /1 The BD Horizon™ Tour

Revealing What is Hidden CD 19→ The most basic information to be derived from any flow cytometry experiment is whether the cells of CD 8→ interest are positive for a given marker Flow cytometry’s major challenge is revealing dim events / populations which are hidden in the background. The BD Horizon™ Tour: New insights for multicolor panel design /2 CD 25→

What are TWO major considerations BEFORE embarking on Multicolor panel design? Experimental Goal/Question Instrument Configurations and Capabilities The BD Horizon™ Tour: New insights for multicolor panel design /3

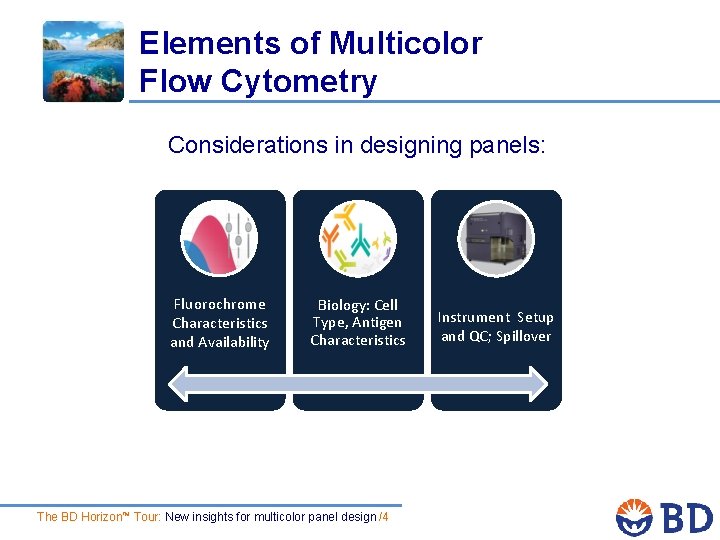
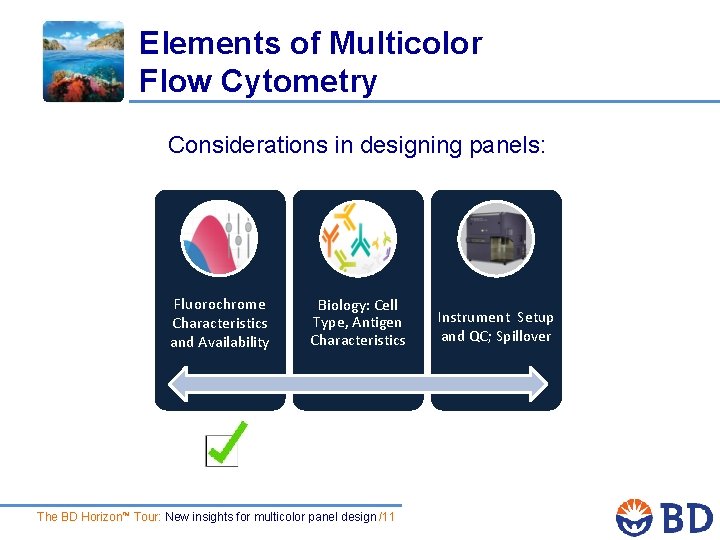
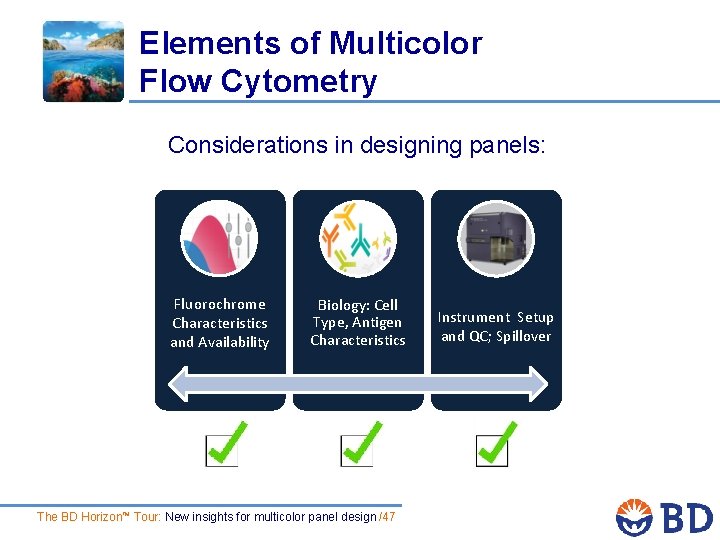
Elements of Multicolor Flow Cytometry Considerations in designing panels: Fluorochrome Characteristics and Availability Biology: Cell Type, Antigen Characteristics The BD Horizon™ Tour: New insights for multicolor panel design /4 Instrument Setup and QC; Spillover

Fluorochromes Expanded range of choices to reveal biological context bdbiosciences. com /5 The BD Horizon™ Tour

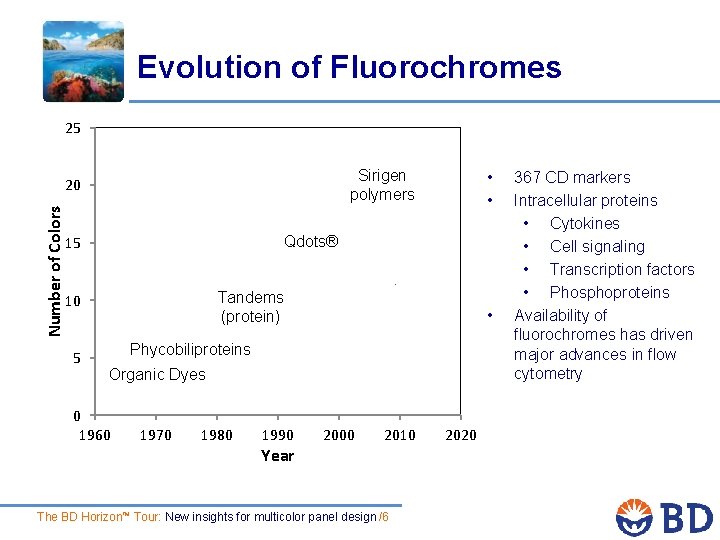
Evolution of Fluorochromes 25 Sirigen polymers Number of Colors 20 Qdots® 15 Tandems (protein) 10 5 • • • Phycobiliproteins Organic Dyes 0 1960 1970 1980 1990 Year 2000 2010 The BD Horizon™ Tour: New insights for multicolor panel design /6 2020 367 CD markers Intracellular proteins • Cytokines • Cell signaling • Transcription factors • Phosphoproteins Availability of fluorochromes has driven major advances in flow cytometry

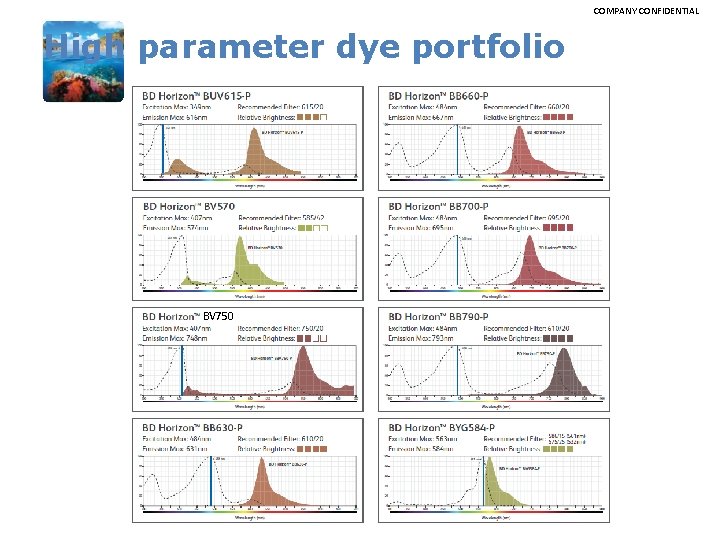
COMPANY CONFIDENTIAL High parameter dye portfolio BV 750

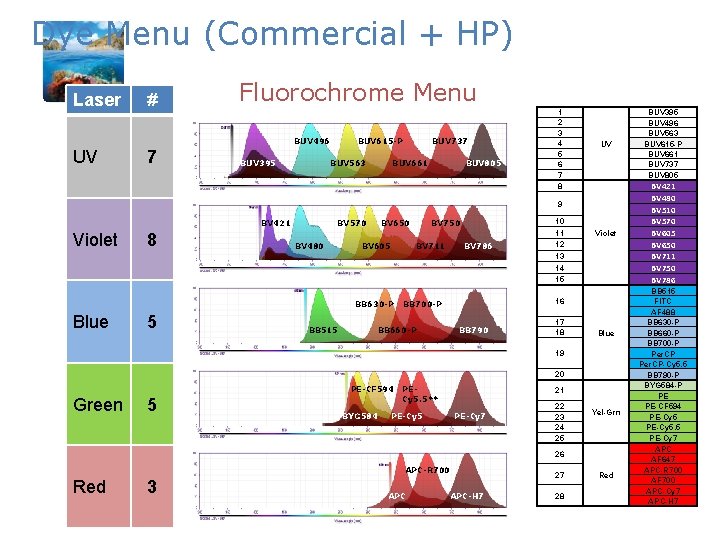
Dye Menu (Commercial + HP) Laser # Fluorochrome Menu BUV 496 UV 7 BUV 395 BUV 615 -P BUV 563 BUV 737 BUV 661 BUV 805 1 2 3 4 5 6 7 8 UV BUV 395 BUV 496 BUV 563 BUV 615 -P BUV 661 BUV 737 BUV 805 Violet BV 421 BV 480 BV 510 BV 570 BV 605 BV 650 BV 711 BV 750 BV 786 9 BV 421 Violet 8 BV 570 BV 480 BV 650 BV 605 BV 711 BB 630 -P Blue 5 BB 515 BV 750 BV 786 10 11 12 13 14 15 16 BB 700 -P BB 660 -P BB 790 17 18 Blue 19 20 PE-CF 594 Green 5 BYG 584 PECy 5. 5** 21 PE-Cy 5 PE-Cy 7 22 23 24 25 Yel-Grn 26 APC-R 700 Red 3 APC-H 7 27 28 Red BB 515 FITC AF 488 BB 630 -P BB 660 -P BB 700 -P Per. CP-Cy 5. 5 BB 790 -P BYG 584 -P PE PE-CF 594 PE-Cy 5. 5 PE-Cy 7 APC AF 647 APC-R 700 AF 700 APC-Cy 7 APC-H 7

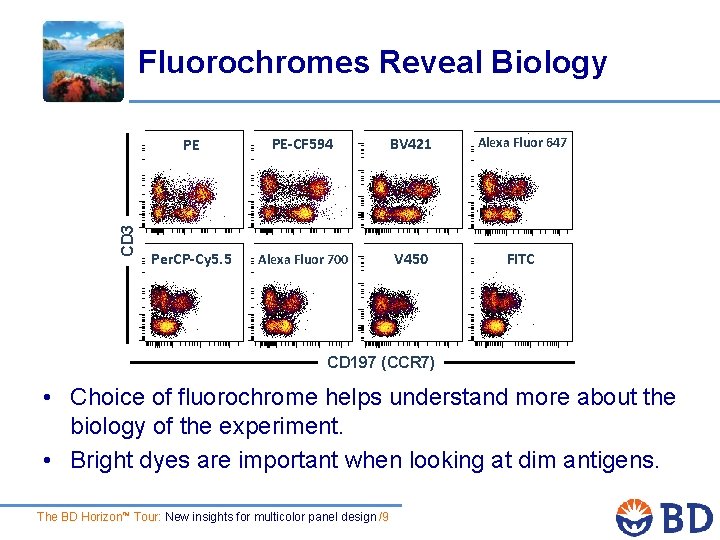
CD 3 Fluorochromes Reveal Biology PE PE-CF 594 BV 421 Alexa Fluor 647 Per. CP-Cy 5. 5 Alexa Fluor 700 V 450 FITC CD 197 (CCR 7) • Choice of fluorochrome helps understand more about the biology of the experiment. • Bright dyes are important when looking at dim antigens. The BD Horizon™ Tour: New insights for multicolor panel design /9

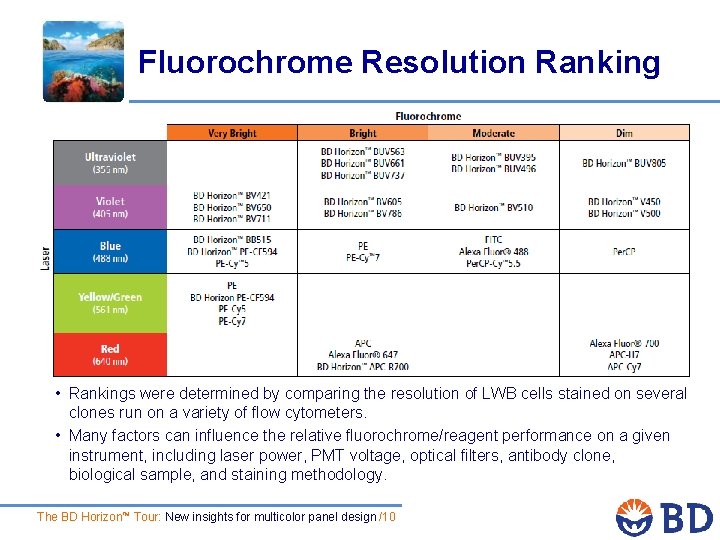
Fluorochrome Resolution Ranking • Rankings were determined by comparing the resolution of LWB cells stained on several clones run on a variety of flow cytometers. • Many factors can influence the relative fluorochrome/reagent performance on a given instrument, including laser power, PMT voltage, optical filters, antibody clone, biological sample, and staining methodology. The BD Horizon™ Tour: New insights for multicolor panel design /10

Elements of Multicolor Flow Cytometry Considerations in designing panels: Fluorochrome Characteristics and Availability Biology: Cell Type, Antigen Characteristics The BD Horizon™ Tour: New insights for multicolor panel design /11 Instrument Setup and QC; Spillover

Poll 1. Are you currently using Antigen Density information to help you build your panels? 2. How are you currently getting information about Antigen expression characteristics to inform multi-color panel design? The BD Horizon™ Tour: New insights for multicolor panel design /12

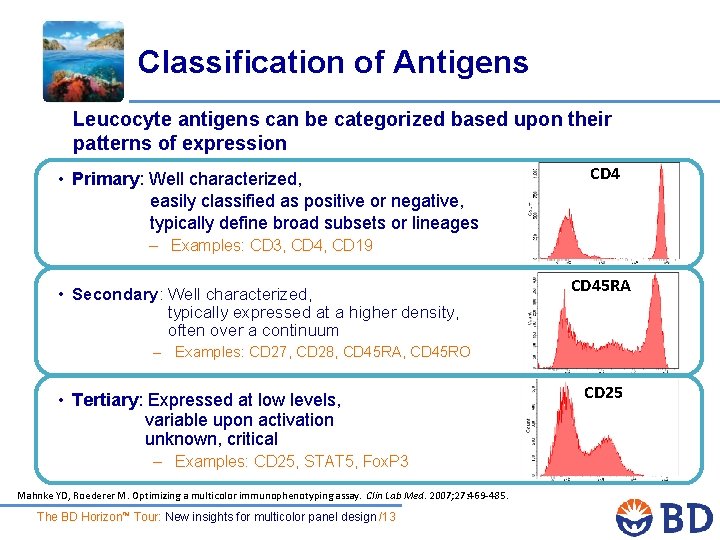
Classification of Antigens Leucocyte antigens can be categorized based upon their patterns of expression CD 4 • Primary: Well characterized, easily classified as positive or negative, typically define broad subsets or lineages – Examples: CD 3, CD 4, CD 19 CD 45 RA • Secondary: Well characterized, typically expressed at a higher density, often over a continuum – Examples: CD 27, CD 28, CD 45 RA, CD 45 RO • Tertiary: Expressed at low levels, variable upon activation unknown, critical CD 25 – Examples: CD 25, STAT 5, Fox. P 3 Mahnke YD, Roederer M. Optimizing a multicolor immunophenotyping assay. Clin Lab Med. 2007; 27: 469 -485. The BD Horizon™ Tour: New insights for multicolor panel design /13

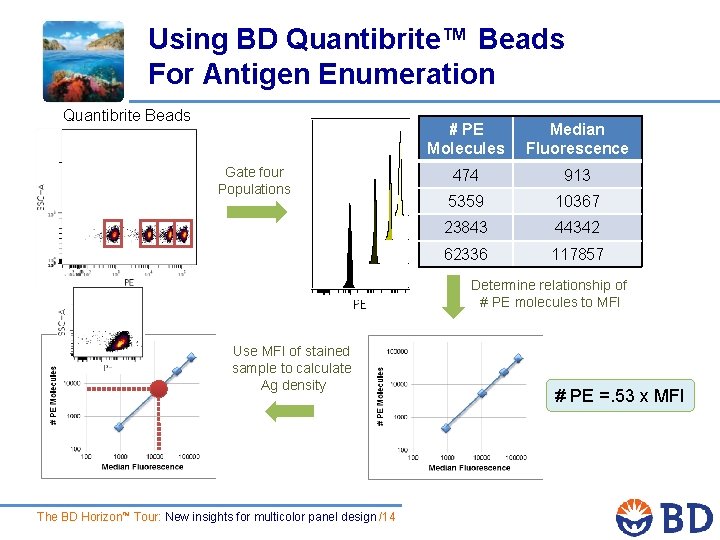
Using BD Quantibrite™ Beads For Antigen Enumeration Quantibrite Beads Gate four Populations # PE Molecules Median Fluorescence 474 913 5359 10367 23843 44342 62336 117857 Determine relationship of # PE molecules to MFI Use MFI of stained sample to calculate Ag density The BD Horizon™ Tour: New insights for multicolor panel design /14 # PE =. 53 x MFI

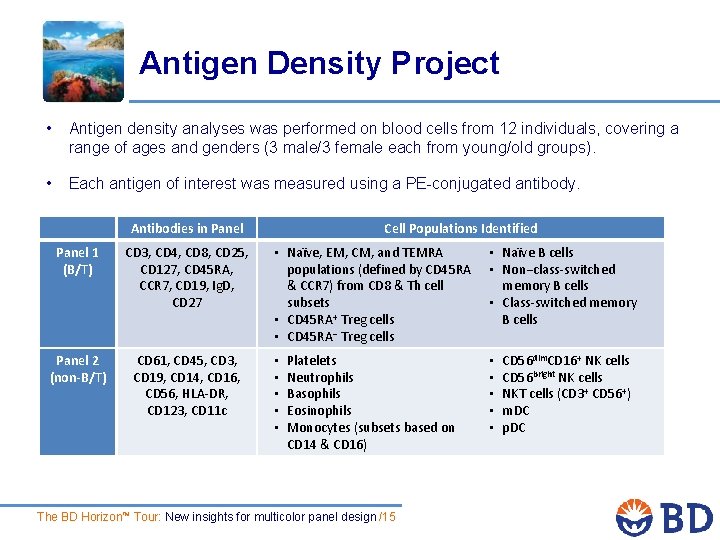
Antigen Density Project • Antigen density analyses was performed on blood cells from 12 individuals, covering a range of ages and genders (3 male/3 female each from young/old groups). • Each antigen of interest was measured using a PE-conjugated antibody. Antibodies in Panel 1 (B/T) CD 3, CD 4, CD 8, CD 25, CD 127, CD 45 RA, CCR 7, CD 19, Ig. D, CD 27 Panel 2 (non-B/T) CD 61, CD 45, CD 3, CD 19, CD 14, CD 16, CD 56, HLA-DR, CD 123, CD 11 c Cell Populations Identified • Naïve, EM, CM, and TEMRA • Naïve B cells populations (defined by CD 45 RA • Non−class-switched & CCR 7) from CD 8 & Th cell memory B cells subsets • Class-switched memory + • CD 45 RA Treg cells B cells • CD 45 RA− Treg cells • • • Platelets Neutrophils Basophils Eosinophils Monocytes (subsets based on CD 14 & CD 16) The BD Horizon™ Tour: New insights for multicolor panel design /15 • • • CD 56 dim. CD 16+ NK cells CD 56 bright NK cells NKT cells (CD 3+ CD 56+) m. DC p. DC

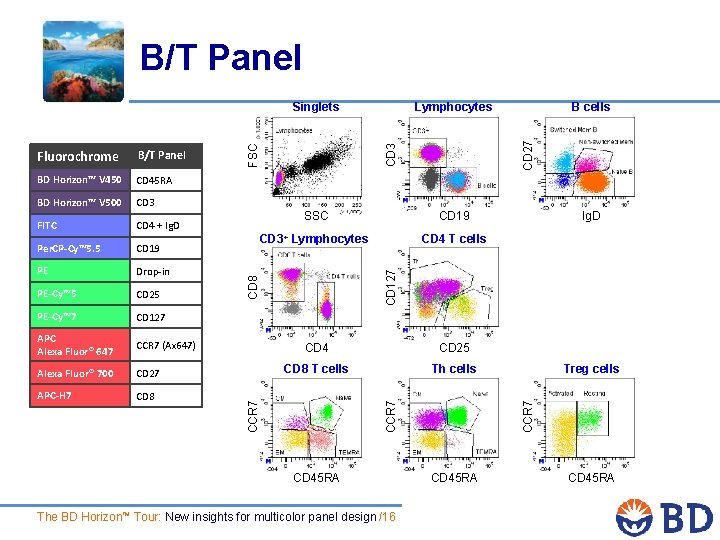
B/T Panel CD 4 + Ig. D Per. CP-Cy™ 5. 5 CD 19 PE Drop-in PE-Cy™ 5 CD 25 PE-Cy™ 7 CD 127 APC Alexa Fluor® 647 CCR 7 (Ax 647) Alexa Fluor® 700 CD 27 APC-H 7 CD 8 CD 27 FITC SSC CD 19 CD 3+ Lymphocytes CD 4 T cells Ig. D CD 4 CD 25 CD 8 T cells Th cells CD 45 RA The BD Horizon™ Tour: New insights for multicolor panel design /16 Treg cells CCR 7 CD 3 B cells CD 127 BD Horizon™ V 500 CCR 7 CD 45 RA FSC BD Horizon™ V 450 CD 8 B/T Panel CCR 7 Fluorochrome Lymphocytes CD 3 Singlets CD 45 RA

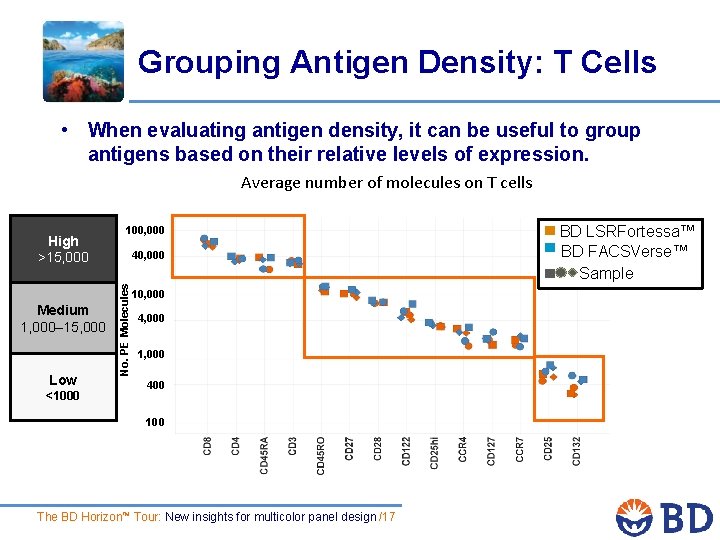
Grouping Antigen Density: T Cells • When evaluating antigen density, it can be useful to group antigens based on their relative levels of expression. Average number of molecules on T cells Medium 1, 000– 15, 000 Low <1000 100, 000 40, 000 No. PE Molecules High >15, 000 10, 000 4, 000 1, 000 400 100 The BD Horizon™ Tour: New insights for multicolor panel design /17 BD LSRFortessa™ BD FACSVerse™ Sample

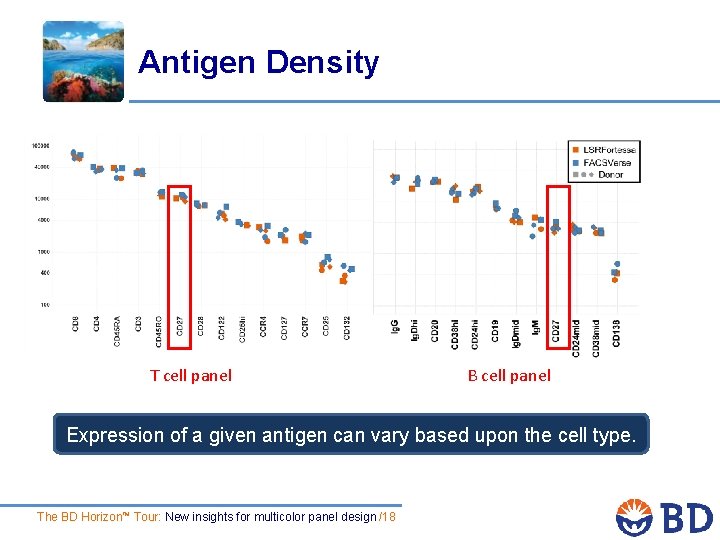
Antigen Density T cell panel B cell panel Expression of a given antigen can vary based upon the cell type. The BD Horizon™ Tour: New insights for multicolor panel design /18

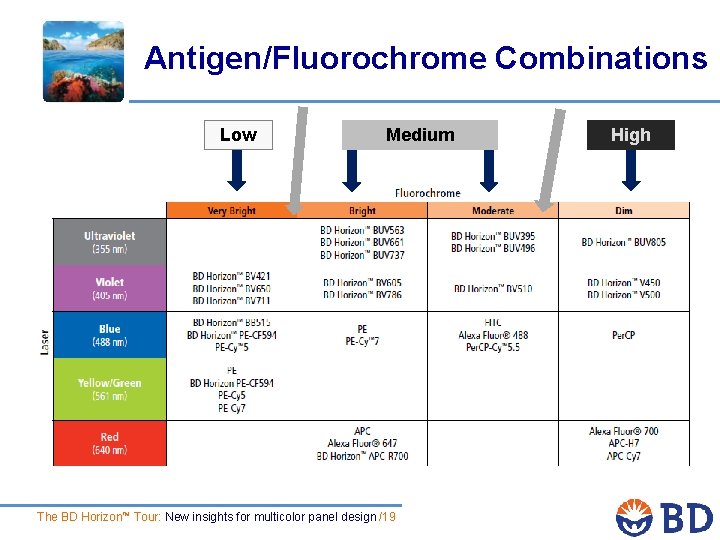
Antigen/Fluorochrome Combinations Low Medium The BD Horizon™ Tour: New insights for multicolor panel design /19 High

Elements of Multicolor Flow Cytometry Considerations in designing panels: Fluorochrome Characteristics and Availability Biology: Cell Type, Antigen Characteristics The BD Horizon™ Tour: New insights for multicolor panel design /20 Instrument Setup and QC; Spillover

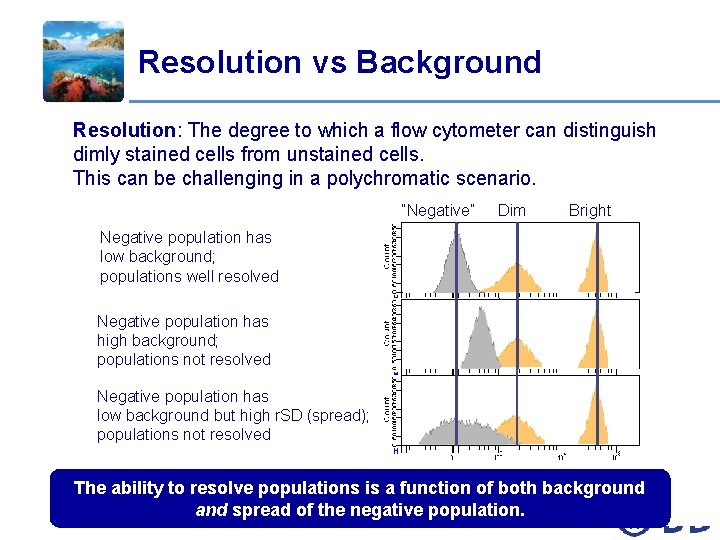
Resolution vs Background Resolution: The degree to which a flow cytometer can distinguish dimly stained cells from unstained cells. This can be challenging in a polychromatic scenario. “Negative” Dim Bright Negative population has low background; populations well resolved Negative population has high background; populations not resolved Negative population has low background but high r. SD (spread); populations not resolved The ability to resolve populations is a function of both background and spread of the negative population. The BD Horizon™ Tour: New insights for multicolor panel design /21

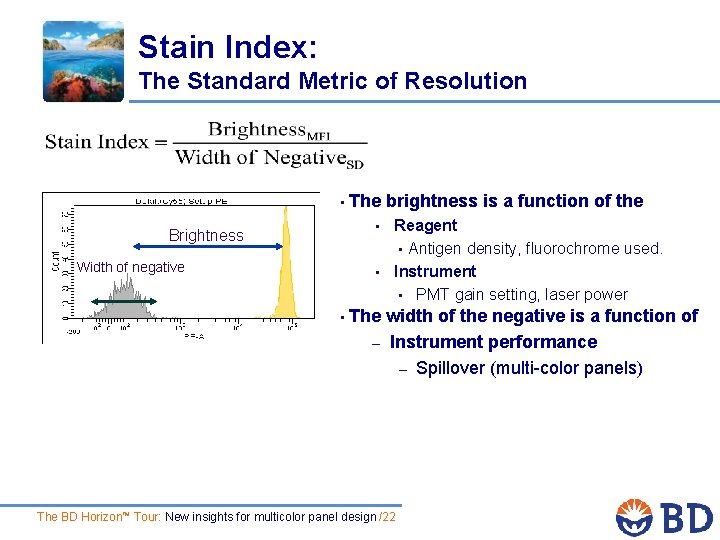
Stain Index: The Standard Metric of Resolution • The brightness is a function of the • Brightness Width of negative • • Reagent • Antigen density, fluorochrome used. Instrument • PMT gain setting, laser power The width of the negative is a function of – Instrument performance – Spillover (multi-color panels) The BD Horizon™ Tour: New insights for multicolor panel design /22

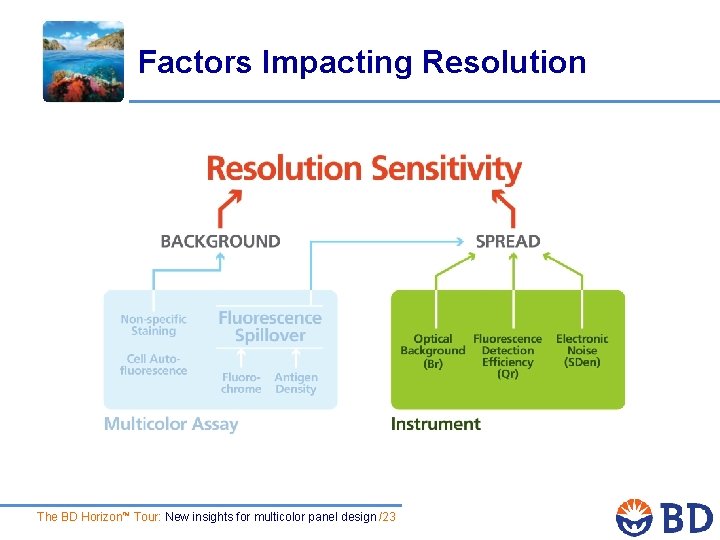
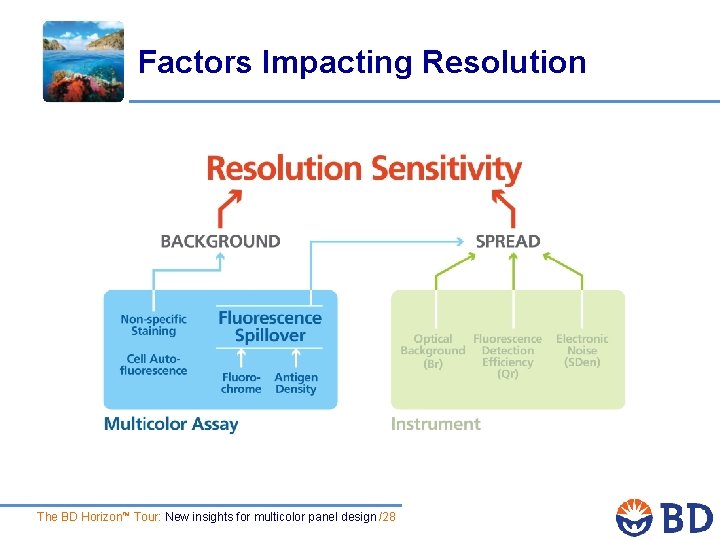
Factors Impacting Resolution The BD Horizon™ Tour: New insights for multicolor panel design /23

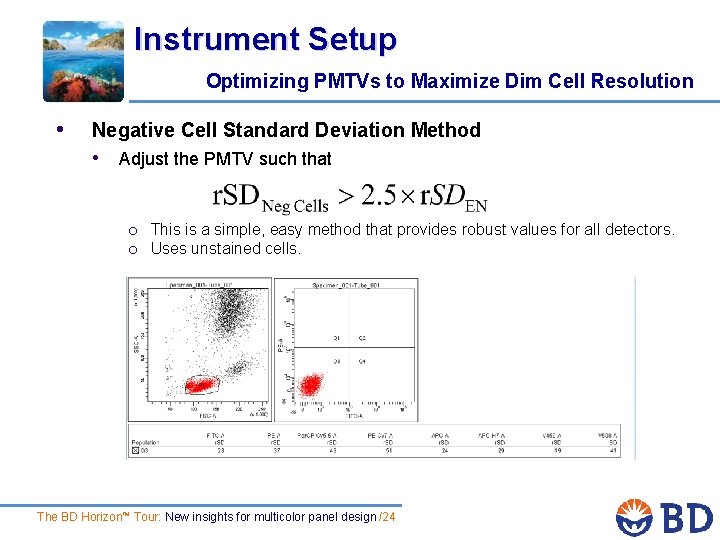
Instrument Setup Optimizing PMTVs to Maximize Dim Cell Resolution • Negative Cell Standard Deviation Method • Adjust the PMTV such that o This is a simple, easy method that provides robust values for all detectors. o Uses unstained cells. The BD Horizon™ Tour: New insights for multicolor panel design /24

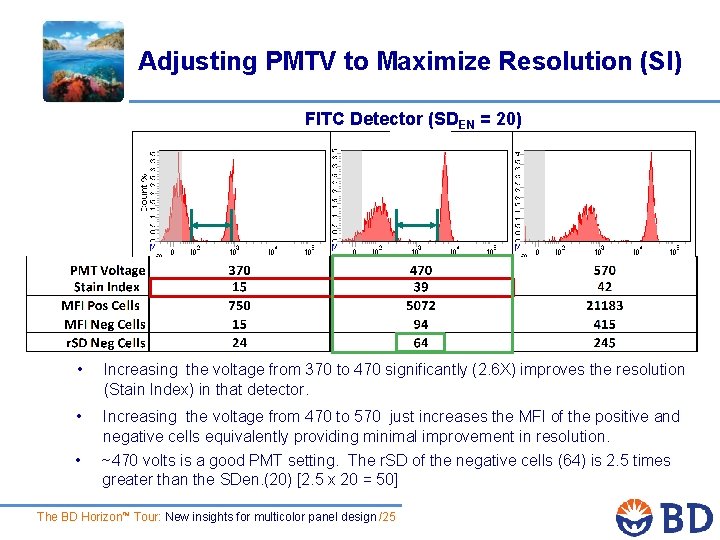
Adjusting PMTV to Maximize Resolution (SI) FITC Detector (SDEN = 20) r. SD is > 2. 5 SDen (~20) • Increasing the voltage from 370 to 470 significantly (2. 6 X) improves the resolution (Stain Index) in that detector. • Increasing the voltage from 470 to 570 just increases the MFI of the positive and negative cells equivalently providing minimal improvement in resolution. • ~470 volts is a good PMT setting. The r. SD of the negative cells (64) is 2. 5 times greater than the SDen. (20) [2. 5 x 20 = 50] The BD Horizon™ Tour: New insights for multicolor panel design /25

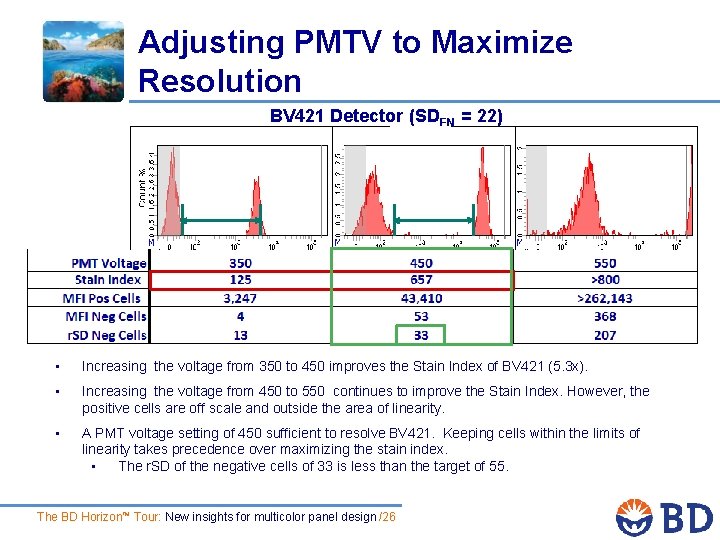
Adjusting PMTV to Maximize Resolution BV 421 Detector (SDEN = 22) • Increasing the voltage from 350 to 450 improves the Stain Index of BV 421 (5. 3 x). • Increasing the voltage from 450 to 550 continues to improve the Stain Index. However, the positive cells are off scale and outside the area of linearity. • A PMT voltage setting of 450 sufficient to resolve BV 421. Keeping cells within the limits of linearity takes precedence over maximizing the stain index. • The r. SD of the negative cells of 33 is less than the target of 55. The BD Horizon™ Tour: New insights for multicolor panel design /26

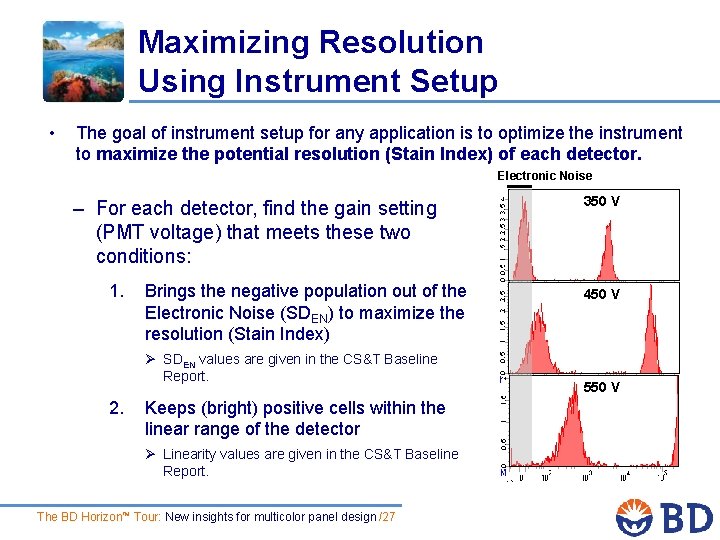
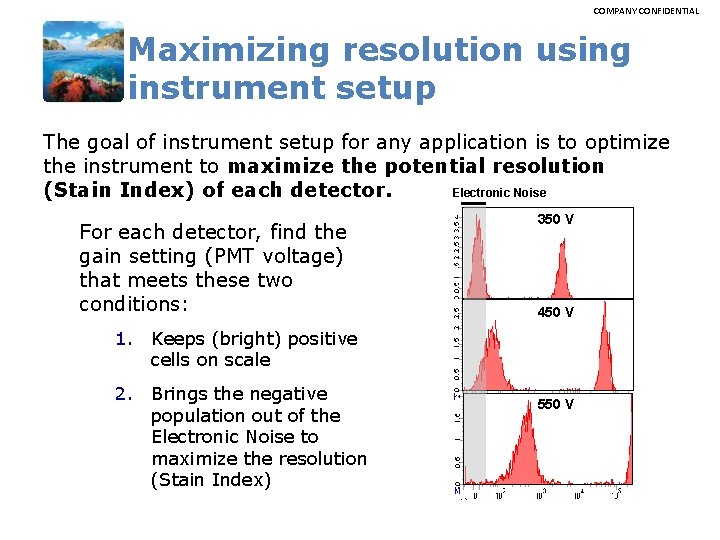
Maximizing Resolution Using Instrument Setup • The goal of instrument setup for any application is to optimize the instrument to maximize the potential resolution (Stain Index) of each detector. Electronic Noise – For each detector, find the gain setting (PMT voltage) that meets these two conditions: 1. Brings the negative population out of the Electronic Noise (SDEN) to maximize the resolution (Stain Index) Ø SDEN values are given in the CS&T Baseline Report. 2. Keeps (bright) positive cells within the linear range of the detector Ø Linearity values are given in the CS&T Baseline Report. The BD Horizon™ Tour: New insights for multicolor panel design /27 350 V 450 V 550 V

Factors Impacting Resolution The BD Horizon™ Tour: New insights for multicolor panel design /28

Fluorescence Spillover § Is the single most important factor affecting resolution sensitivity (SI) in multicolor flow cytometry experiments § Fluorescence spillover from other channels: • Directly and irreversibly reduces the resolution sensitivity of that channel • Contributes to background § This “background” is subtracted in the process called compensation. Automatic compensation in Diva The BD Horizon™ Tour: New insights for multicolor panel design /29

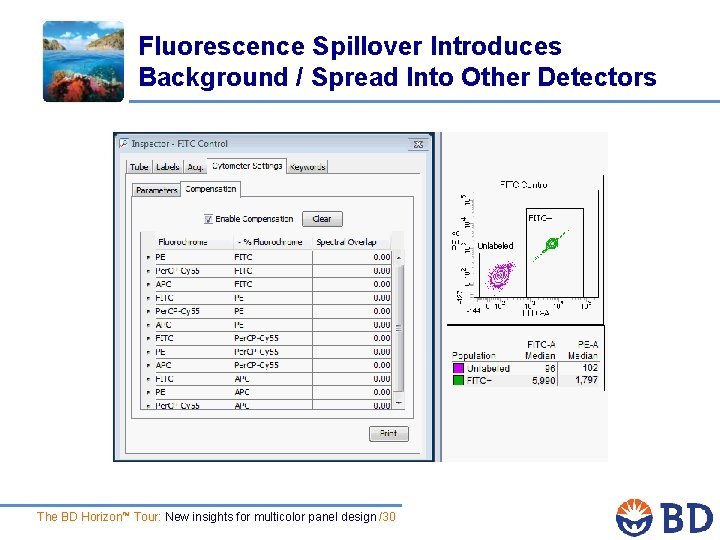
Fluorescence Spillover Introduces Background / Spread Into Other Detectors The BD Horizon™ Tour: New insights for multicolor panel design /30

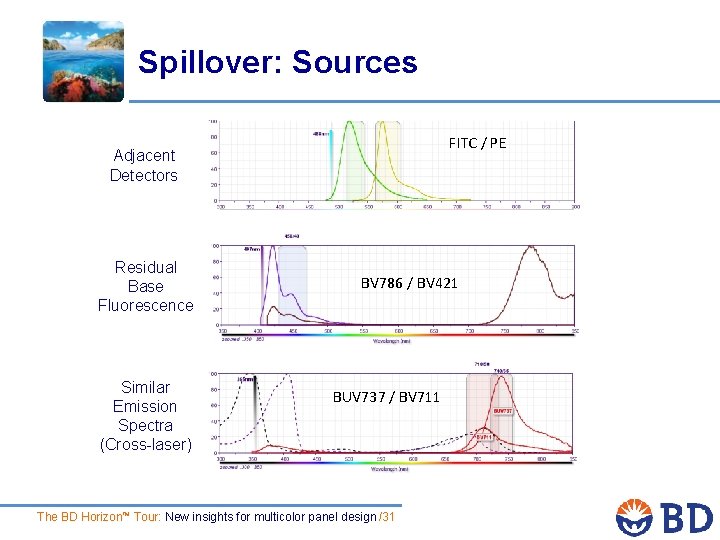
Spillover: Sources FITC / PE Adjacent Detectors Residual Base Fluorescence Similar Emission Spectra (Cross-laser) BV 786 / BV 421 BUV 737 / BV 711 The BD Horizon™ Tour: New insights for multicolor panel design /31

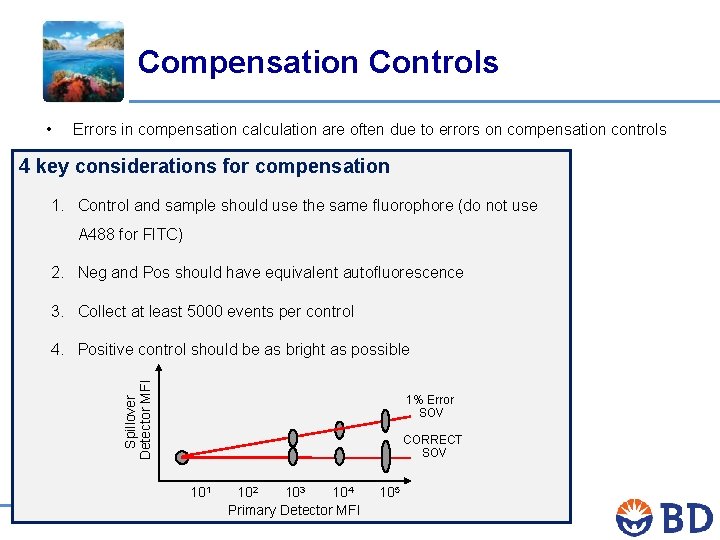
Compensation Controls • Errors in compensation calculation are often due to errors on compensation controls 4 key considerations for compensation 1. Control and sample should use the same fluorophore (do not use A 488 for FITC) 2. Neg and Pos should have equivalent autofluorescence 3. Collect at least 5000 events per control Spillover Detector MFI 4. Positive control should be as bright as possible 1% Error SOV CORRECT SOV 105 104 102 103 Primary Detector MFI The BD Horizon™ Tour: New insights for multicolor panel design /32 101

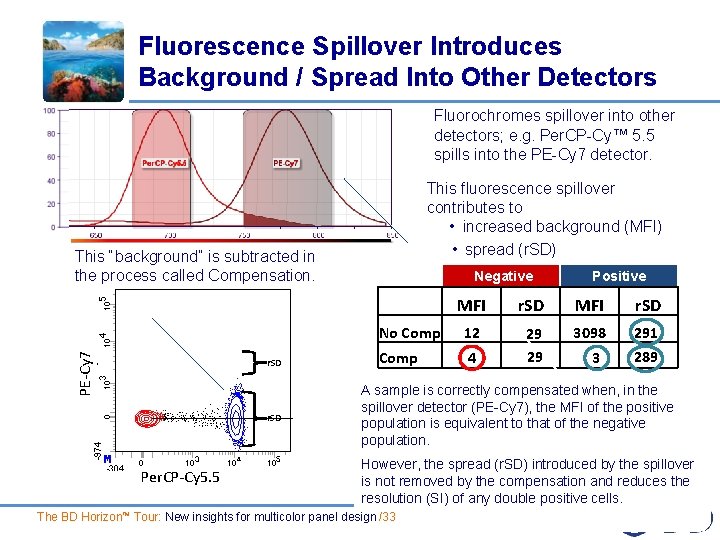
Fluorescence Spillover Introduces Background / Spread Into Other Detectors Fluorochromes spillover into other detectors; e. g. Per. CP-Cy™ 5. 5 spills into the PE-Cy 7 detector. This “background” is subtracted in the process called Compensation. This fluorescence spillover contributes to • increased background (MFI) • spread (r. SD) PE-Cy 7 r. SD Per. CP-Cy 5. 5 Negative MFI No Comp 12 Comp 4 r. SD Positive MFI r. SD 3098 29 29 3 291 289 A sample is correctly compensated when, in the spillover detector (PE-Cy 7), the MFI of the positive population is equivalent to that of the negative population. However, the spread (r. SD) introduced by the spillover is not removed by the compensation and reduces the resolution (SI) of any double positive cells. The BD Horizon™ Tour: New insights for multicolor panel design /33

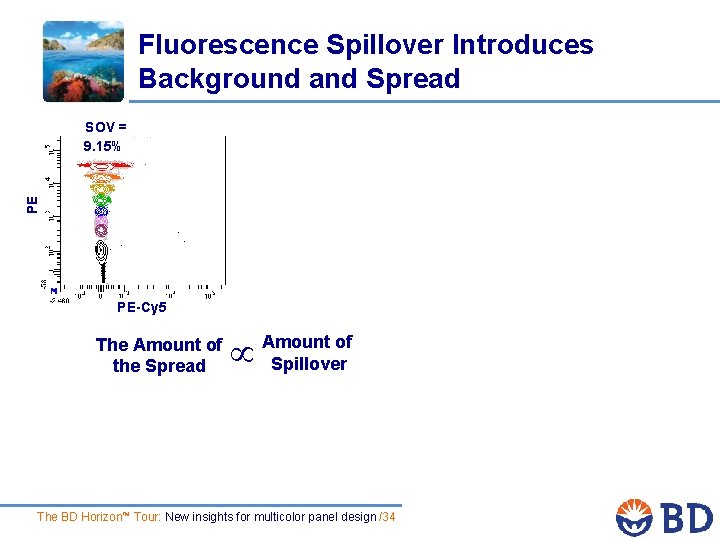
Fluorescence Spillover Introduces Background and Spread PE SOV = 9. 15% PE-Cy 5 The Amount of the Spread Amount of Spillover The BD Horizon™ Tour: New insights for multicolor panel design /34

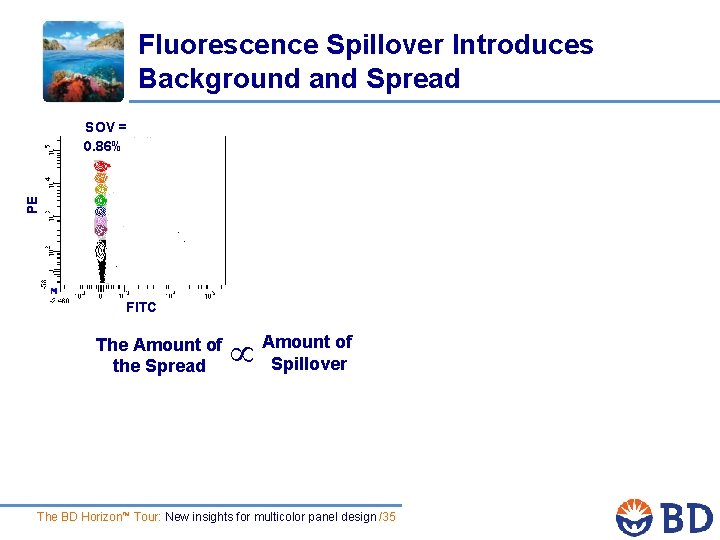
Fluorescence Spillover Introduces Background and Spread PE SOV = 0. 86% FITC The Amount of the Spread Amount of Spillover The BD Horizon™ Tour: New insights for multicolor panel design /35

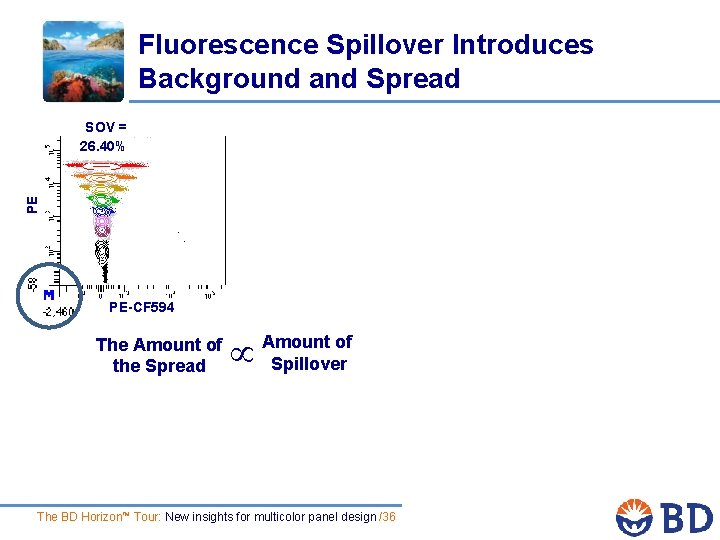
Fluorescence Spillover Introduces Background and Spread PE SOV = 26. 40% PE-CF 594 The Amount of the Spread Amount of Spillover The BD Horizon™ Tour: New insights for multicolor panel design /36

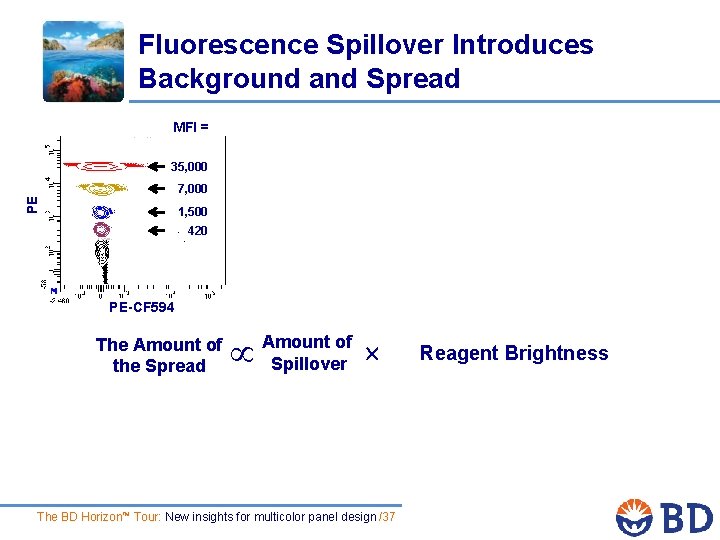
Fluorescence Spillover Introduces Background and Spread MFI = 35, 000 PE 7, 000 1, 500 420 PE-CF 594 The Amount of the Spread Amount of Spillover The BD Horizon™ Tour: New insights for multicolor panel design /37 Reagent Brightness

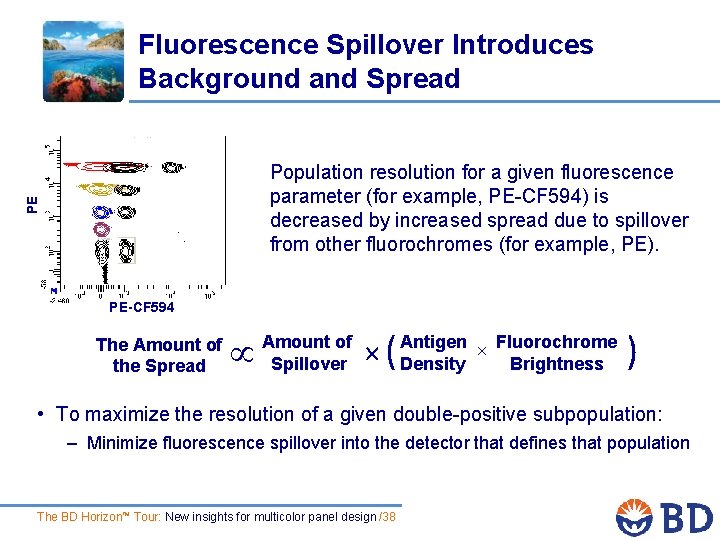
Fluorescence Spillover Introduces Background and Spread PE Population resolution for a given fluorescence parameter (for example, PE-CF 594) is decreased by increased spread due to spillover from other fluorochromes (for example, PE). PE-CF 594 The Amount of the Spread Amount of Spillover ( Antigen Fluorochrome Density Brightness ) • To maximize the resolution of a given double-positive subpopulation: – Minimize fluorescence spillover into the detector that defines that population The BD Horizon™ Tour: New insights for multicolor panel design /38

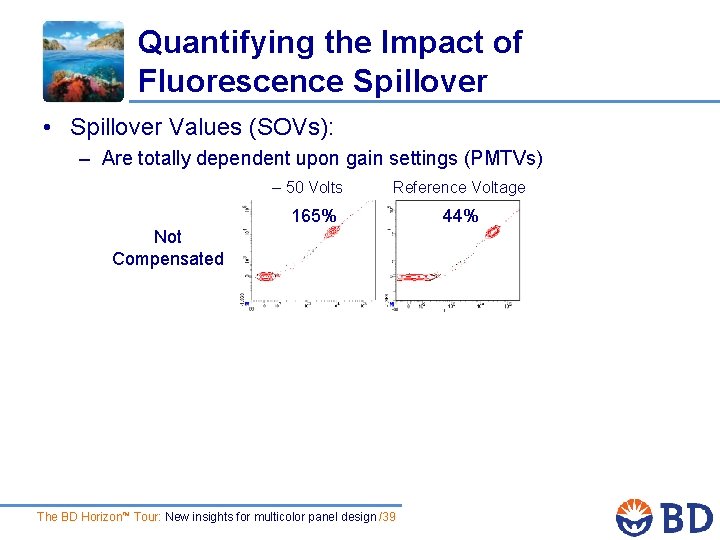
Quantifying the Impact of Fluorescence Spillover • Spillover Values (SOVs): – Are totally dependent upon gain settings (PMTVs) – 50 Volts Reference Voltage 165% Not Compensated The BD Horizon™ Tour: New insights for multicolor panel design /39 44%

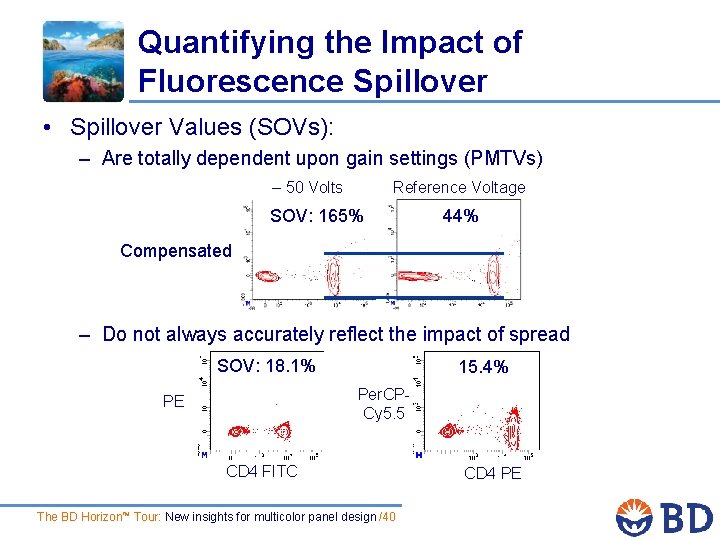
Quantifying the Impact of Fluorescence Spillover • Spillover Values (SOVs): – Are totally dependent upon gain settings (PMTVs) – 50 Volts Reference Voltage SOV: 165% 44% Compensated – Do not always accurately reflect the impact of spread SOV: 18. 1% 15. 4% Per. CPCy 5. 5 PE CD 4 FITC The BD Horizon™ Tour: New insights for multicolor panel design /40 CD 4 PE

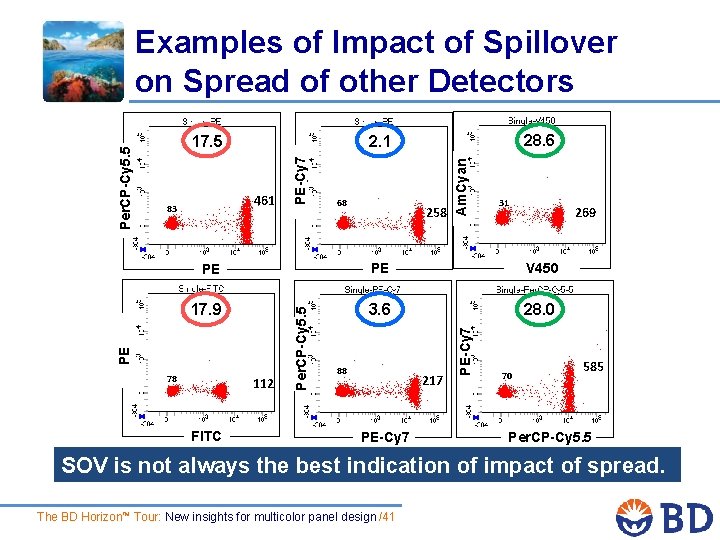
68 258 Am. Cyan 461 83 28. 6 2. 1 PE-Cy 7 17. 5 31 269 V 450 17. 9 3. 6 28. 0 78 112 FITC 88 217 PE-Cy 7 PE Per. CP-Cy 5. 5 PE PE Per. CP-Cy 5. 5 Examples of Impact of Spillover on Spread of other Detectors 70 585 Per. CP-Cy 5. 5 SOV is not always the best indication of impact of spread. The BD Horizon™ Tour: New insights for multicolor panel design /41

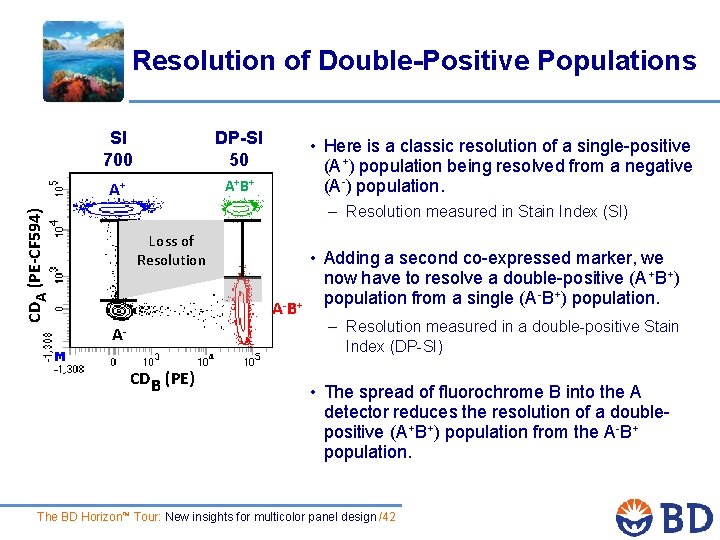
Resolution of Double-Positive Populations SI 700 DP-SI 50 A+ A+B+ • Here is a classic resolution of a single-positive (A+) population being resolved from a negative (A-) population. CDA (PE-CF 594) – Resolution measured in Stain Index (SI) Loss of Resolution A-B+ ACDB (PE) • Adding a second co-expressed marker, we now have to resolve a double-positive (A+B+) population from a single (A-B+) population. – Resolution measured in a double-positive Stain Index (DP-SI) • The spread of fluorochrome B into the A detector reduces the resolution of a doublepositive (A+B+) population from the A-B+ population. The BD Horizon™ Tour: New insights for multicolor panel design /42

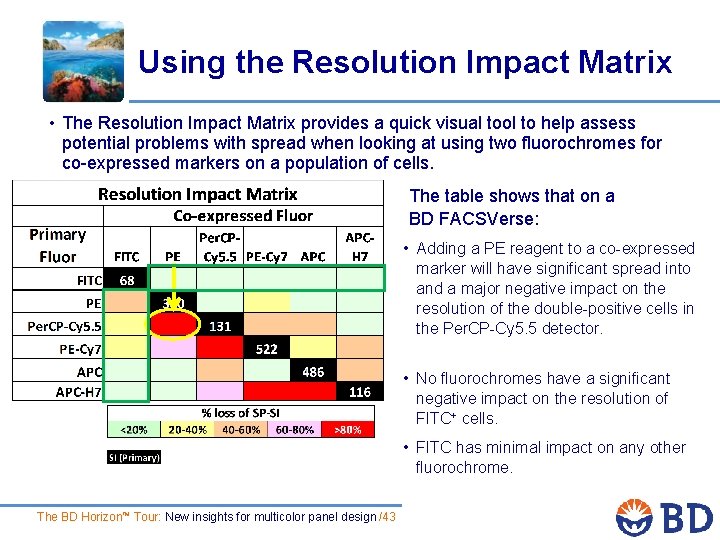
Using the Resolution Impact Matrix • The Resolution Impact Matrix provides a quick visual tool to help assess potential problems with spread when looking at using two fluorochromes for co-expressed markers on a population of cells. The table shows that on a BD FACSVerse: • Adding a PE reagent to a co-expressed marker will have significant spread into and a major negative impact on the resolution of the double-positive cells in the Per. CP-Cy 5. 5 detector. • No fluorochromes have a significant negative impact on the resolution of FITC+ cells. • FITC has minimal impact on any other fluorochrome. The BD Horizon™ Tour: New insights for multicolor panel design /43

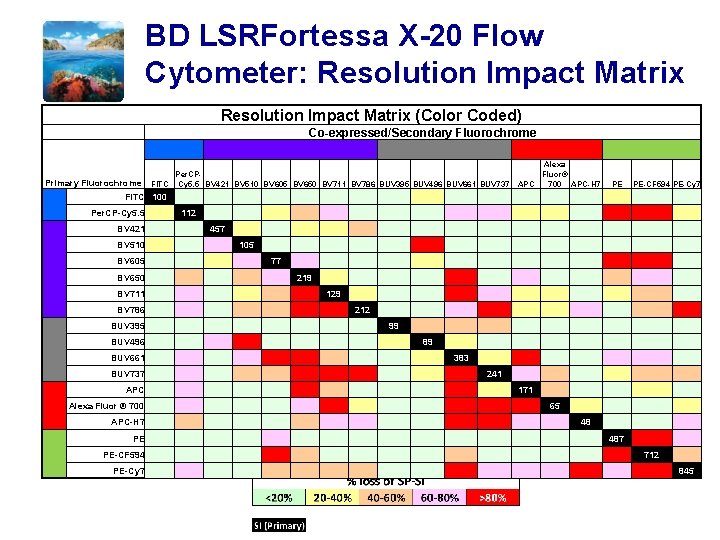
BD LSRFortessa X-20 Flow Cytometer: Resolution Impact Matrix (Color Coded) PE PE-CF 594 PE-Cy 7 112 BV 421 457 BV 510 105 BV 605 77 BV 650 219 BV 711 129 BV 786 212 BUV 395 99 BUV 496 89 BUV 661 383 BUV 737 241 APC 171 Alexa Fluor ® 700 65 APC-H 7 48 PE 487 PE-CF 594 712 PE-Cy 7 845 Per. CP-Cy 5. 5 Alexa Fluor® Per. CPFITC Cy 5. 5 BV 421 BV 510 BV 605 BV 650 BV 711 BV 786 BUV 395 BUV 496 BUV 661 BUV 737 APC 700 APC-H 7 FITC 100 Primary Fluorochrome Co-expressed/Secondary Fluorochrome

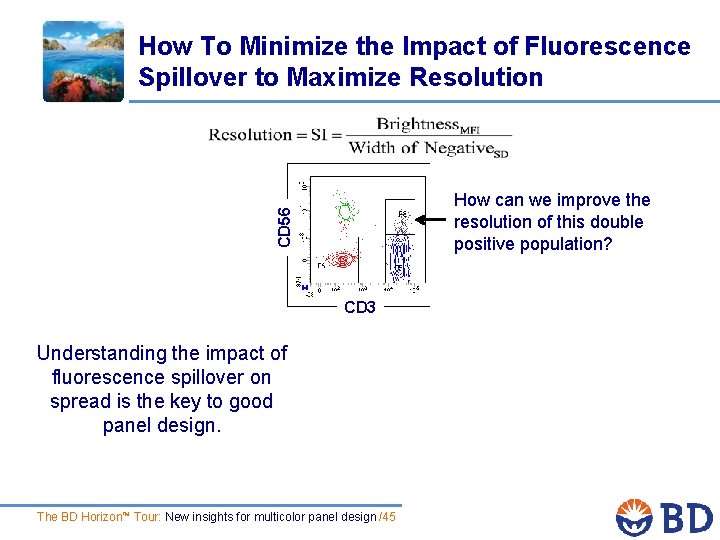
How To Minimize the Impact of Fluorescence Spillover to Maximize Resolution CD 56 How can we improve the resolution of this double positive population? CD 3 Understanding the impact of fluorescence spillover on spread is the key to good panel design. The BD Horizon™ Tour: New insights for multicolor panel design /45

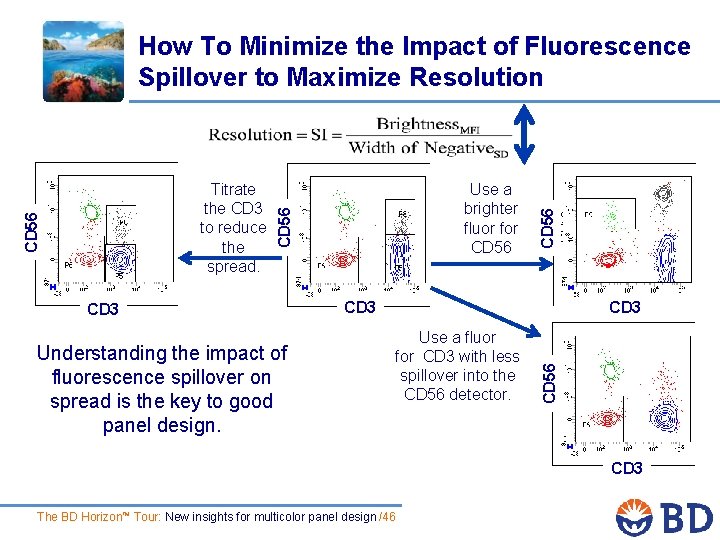
Use a brighter fluor for CD 56 CD 3 Understanding the impact of fluorescence spillover on spread is the key to good panel design. CD 3 Use a fluor for CD 3 with less spillover into the CD 56 detector. CD 56 Titrate the CD 3 to reduce the spread. CD 56 How To Minimize the Impact of Fluorescence Spillover to Maximize Resolution CD 3 The BD Horizon™ Tour: New insights for multicolor panel design /46

Elements of Multicolor Flow Cytometry Considerations in designing panels: Fluorochrome Characteristics and Availability Biology: Cell Type, Antigen Characteristics The BD Horizon™ Tour: New insights for multicolor panel design /47 Instrument Setup and QC; Spillover

Learning Objectives • How to get more information from your assays using experimental panel design • Understanding: – The properties of the fluorochromes to be used • Brightness and spillover – The biology inherent in the assay • Sample type, population of interest, antigen density, and co-expression – The capabilities of instrument that runs the assay • Optical configuration, QC, setup • Applying logical rules to assign fluorochromes to antibody specificities • Why making these choices is likely to be an iterative process The BD Horizon™ Tour: New insights for multicolor panel design /48

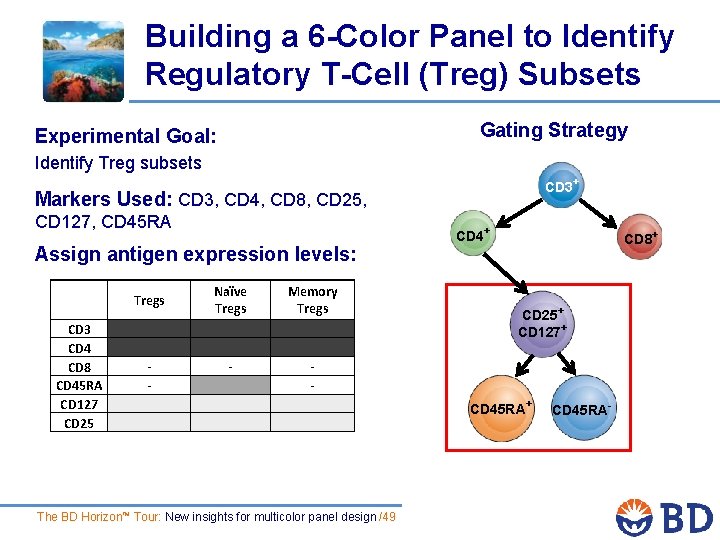
Building a 6 -Color Panel to Identify Regulatory T-Cell (Treg) Subsets Gating Strategy Experimental Goal: Identify Treg subsets CD 3+ Markers Used: CD 3, CD 4, CD 8, CD 25, CD 127, CD 45 RA Assign antigen expression levels: Naïve Tregs CD 3 CD 4 CD 8 CD 45 RA CD 127 CD 25 Memory Tregs - - - CD 4+ CD 8+ CD 25+ CD 127+ CD 45 RA+ The BD Horizon™ Tour: New insights for multicolor panel design /49 CD 45 RA-

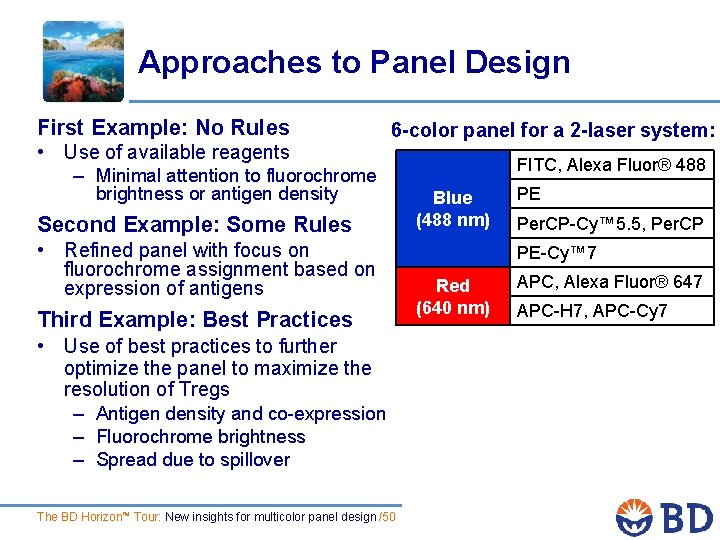
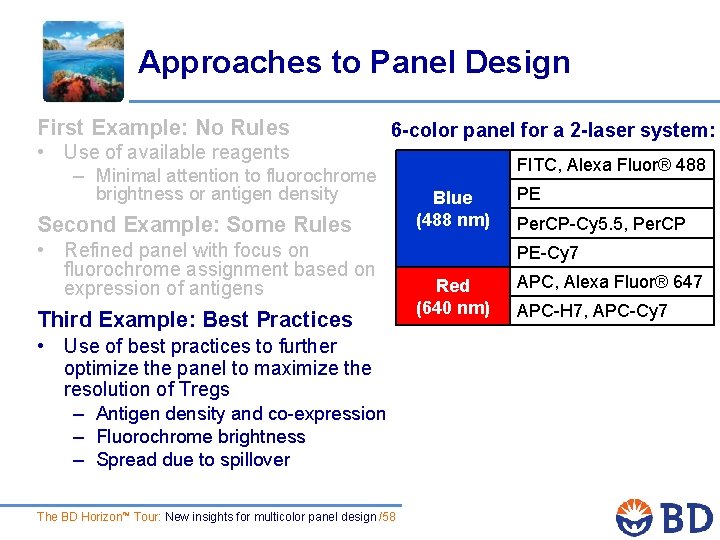
Approaches to Panel Design First Example: No Rules • Use of available reagents – Minimal attention to fluorochrome 6 -color panel for a 2 -laser system: brightness or antigen density Second Example: Some Rules • Refined panel with focus on fluorochrome assignment based on expression of antigens Third Example: Best Practices • Use of best practices to further optimize the panel to maximize the resolution of Tregs – Antigen density and co-expression – Fluorochrome brightness – Spread due to spillover The BD Horizon™ Tour: New insights for multicolor panel design /50 FITC, Alexa Fluor® 488 Blue (488 nm) PE Per. CP-Cy™ 5. 5, Per. CP PE-Cy™ 7 Red (640 nm) APC, Alexa Fluor® 647 APC-H 7, APC-Cy 7

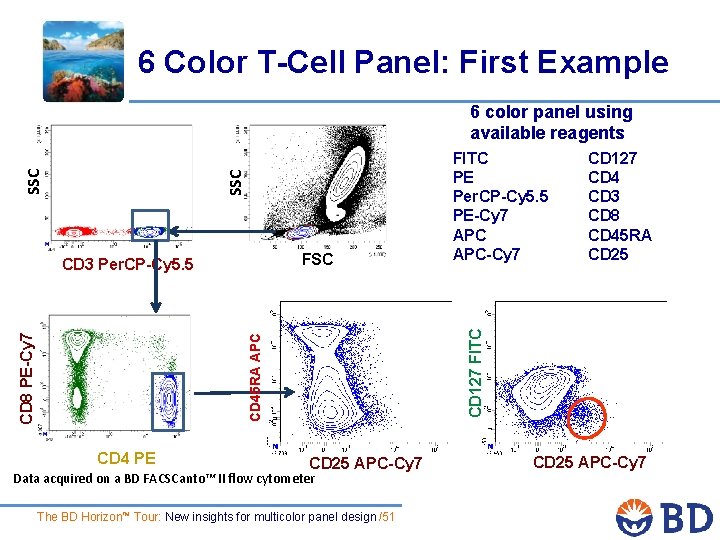
6 Color T-Cell Panel: First Example SSC 6 color panel using available reagents CD 45 RA APC CD 8 PE-Cy 7 CD 4 PE CD 127 CD 4 CD 3 CD 8 CD 45 RA CD 25 CD 127 FITC FSC CD 3 Per. CP-Cy 5. 5 FITC PE Per. CP-Cy 5. 5 PE-Cy 7 APC-Cy 7 CD 25 APC-Cy 7 Data acquired on a BD FACSCanto™ II flow cytometer The BD Horizon™ Tour: New insights for multicolor panel design /51 CD 25 APC-Cy 7

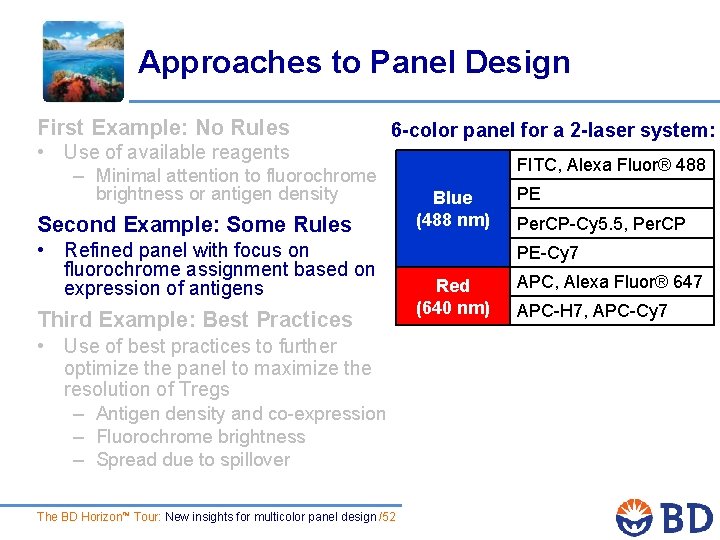
Approaches to Panel Design First Example: No Rules • Use of available reagents – Minimal attention to fluorochrome 6 -color panel for a 2 -laser system: brightness or antigen density Second Example: Some Rules • Refined panel with focus on fluorochrome assignment based on expression of antigens Third Example: Best Practices • Use of best practices to further optimize the panel to maximize the resolution of Tregs – Antigen density and co-expression – Fluorochrome brightness – Spread due to spillover The BD Horizon™ Tour: New insights for multicolor panel design /52 FITC, Alexa Fluor® 488 Blue (488 nm) PE Per. CP-Cy 5. 5, Per. CP PE-Cy 7 Red (640 nm) APC, Alexa Fluor® 647 APC-H 7, APC-Cy 7

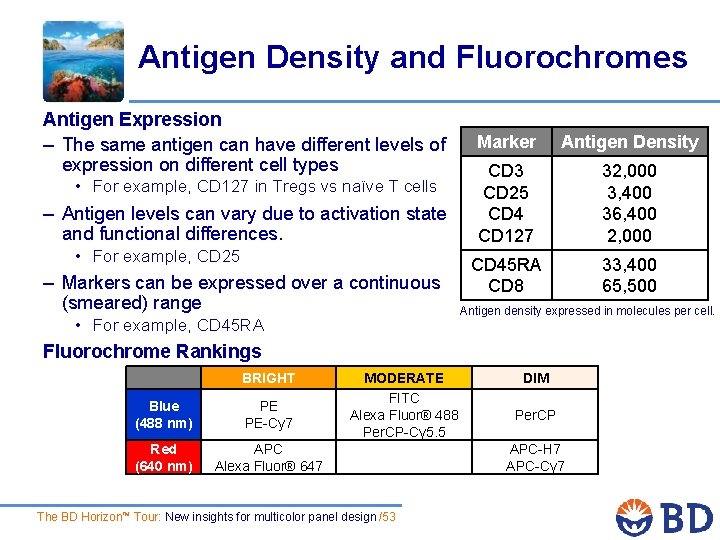
Antigen Density and Fluorochromes Antigen Expression – The same antigen can have different levels of expression on different cell types • For example, CD 127 in Tregs vs naïve T cells – Antigen levels can vary due to activation state and functional differences. • For example, CD 25 – Markers can be expressed over a continuous (smeared) range • For example, CD 45 RA Marker Antigen Density CD 3 CD 25 CD 4 CD 127 32, 000 3, 400 36, 400 2, 000 CD 45 RA CD 8 33, 400 65, 500 Antigen density expressed in molecules per cell. Fluorochrome Rankings BRIGHT Blue (488 nm) PE PE-Cy 7 Red (640 nm) APC Alexa Fluor® 647 MODERATE FITC Alexa Fluor® 488 Per. CP-Cy 5. 5 The BD Horizon™ Tour: New insights for multicolor panel design /53 DIM Per. CP APC-H 7 APC-Cy 7

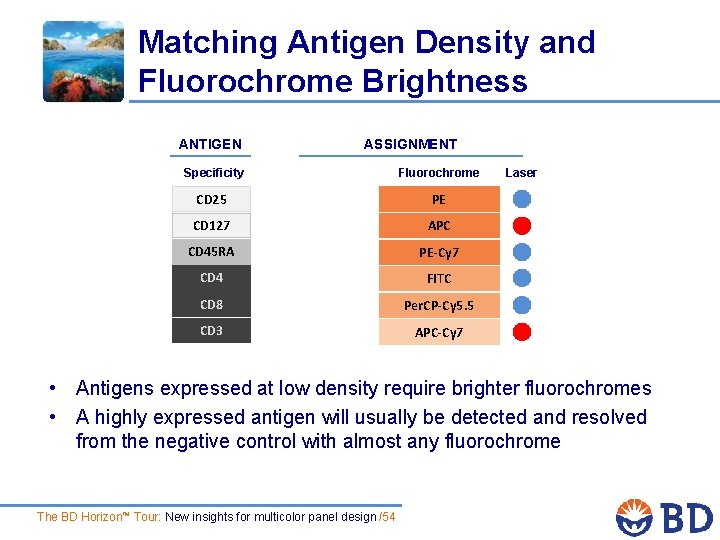
Matching Antigen Density and Fluorochrome Brightness ANTIGEN ASSIGNMENT Specificity Fluorochrome CD 25 PE CD 127 APC CD 45 RA PE-Cy 7 CD 4 FITC CD 8 Per. CP-Cy 5. 5 CD 3 APC-Cy 7 Laser • Antigens expressed at low density require brighter fluorochromes • A highly expressed antigen will usually be detected and resolved from the negative control with almost any fluorochrome The BD Horizon™ Tour: New insights for multicolor panel design /54

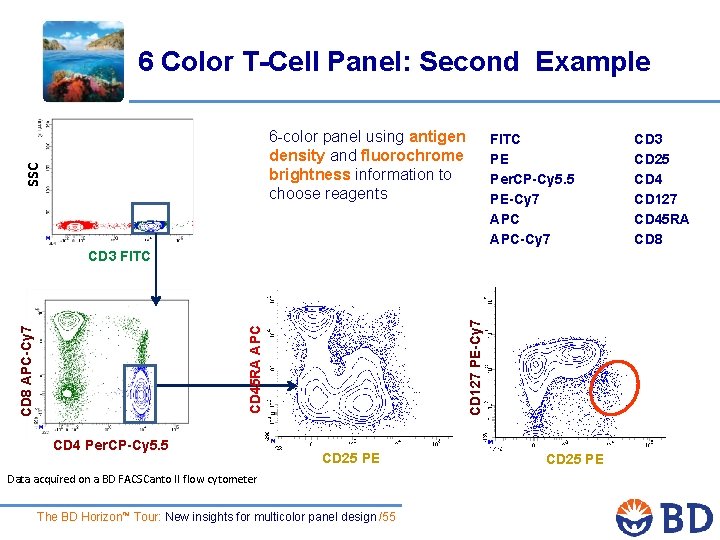
6 Color T-Cell Panel: Second Example SSC 6 -color panel using antigen density and fluorochrome brightness information to choose reagents FITC PE Per. CP-Cy 5. 5 PE-Cy 7 APC-Cy 7 CD 8 APC-Cy 7 CD 45 RA APC CD 127 PE-Cy 7 CD 3 FITC CD 4 Per. CP-Cy 5. 5 CD 25 PE Data acquired on a BD FACSCanto II flow cytometer The BD Horizon™ Tour: New insights for multicolor panel design /55 CD 25 PE CD 3 CD 25 CD 4 CD 127 CD 45 RA CD 8

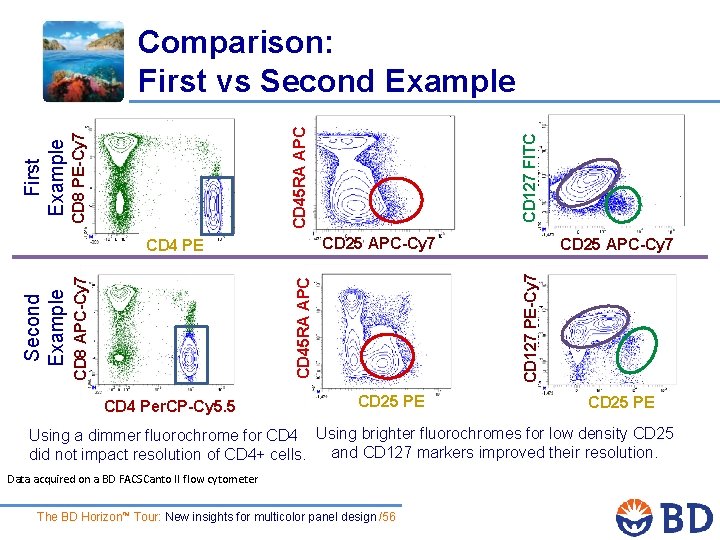
CD 25 APC-Cy 7 CD 4 Per. CP-Cy 5. 5 CD 25 APC-Cy 7 CD 127 PE-Cy 7 CD 45 RA APC CD 8 APC-Cy 7 CD 4 PE Second Example CD 127 FITC CD 45 RA APC CD 8 PE-Cy 7 First Example Comparison: First vs Second Example CD 25 PE Using a dimmer fluorochrome for CD 4 Using brighter fluorochromes for low density CD 25 and CD 127 markers improved their resolution. did not impact resolution of CD 4+ cells. Data acquired on a BD FACSCanto II flow cytometer The BD Horizon™ Tour: New insights for multicolor panel design /56

Quick Quiz It remains challenging to gate on Tregs. Why? – Because spillover and co-expression were not taken into consideration The BD Horizon™ Tour: New insights for multicolor panel design /57

Approaches to Panel Design First Example: No Rules • Use of available reagents – Minimal attention to fluorochrome 6 -color panel for a 2 -laser system: brightness or antigen density Second Example: Some Rules • Refined panel with focus on fluorochrome assignment based on expression of antigens Third Example: Best Practices • Use of best practices to further optimize the panel to maximize the resolution of Tregs – Antigen density and co-expression – Fluorochrome brightness – Spread due to spillover The BD Horizon™ Tour: New insights for multicolor panel design /58 FITC, Alexa Fluor® 488 Blue (488 nm) PE Per. CP-Cy 5. 5, Per. CP PE-Cy 7 Red (640 nm) APC, Alexa Fluor® 647 APC-H 7, APC-Cy 7

Best Practices The key to any final panel optimization is to focus on the critical populations of interest. – The goal is to minimize loss of resolution due to spread from the fluorescence spillover of co-expressed antigens. How to avoid spectral spillover – When antigens are co-expressed on a cell • Avoid significant spillover of a bright marker into a dim marker. • Spread the antigens across as many lasers as possible. – Fluorochromes that are excited by more than one laser cause high spillover. • Am. Cyan excited by the violet and blue lasers spills into the FITC detector. • PE-Cy 5 excited by the blue and red lasers spills into APC detector. – Considerations for tandem dyes • Take into consideration residual donor emission. The BD Horizon™ Tour: New insights for multicolor panel design /59

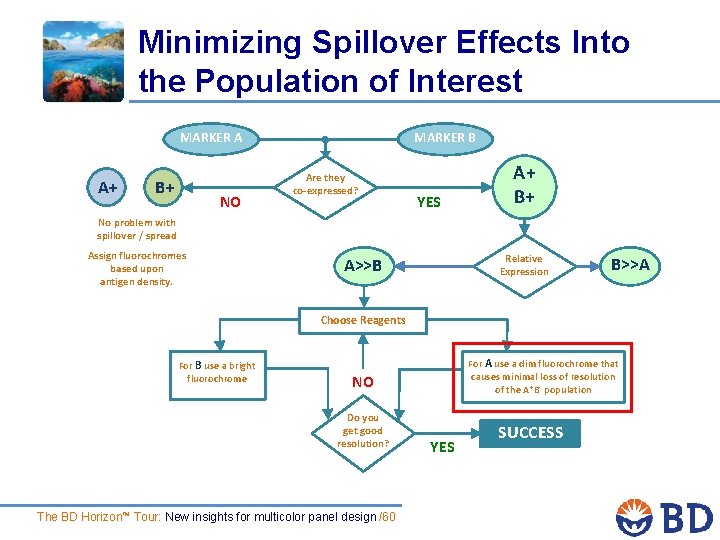
Minimizing Spillover Effects Into the Population of Interest MARKER A A+ B+ NO MARKER B Are they co-expressed? YES A+ B+ No problem with spillover / spread Assign fluorochromes based upon antigen density. Relative Expression A>>B B>>A Choose Reagents For B use a bright fluorochrome For A use a dim fluorochrome that causes minimal loss of resolution of the A+B- population NO Do you get good resolution? The BD Horizon™ Tour: New insights for multicolor panel design /60 YES SUCCESS

Experimental Controls • Controls should be used to help resolve issues in staining. – Isotype controls help identify staining issues. – Unstained controls highlight the background or autofluorescence of the system. – Single-stained controls allow you to QC the compensation and to assess the resolution impact. – Fluorescence Minus One (FMO) controls help identify gating boundaries and illustrate the potential impact of spillover. The BD Horizon™ Tour: New insights for multicolor panel design /61

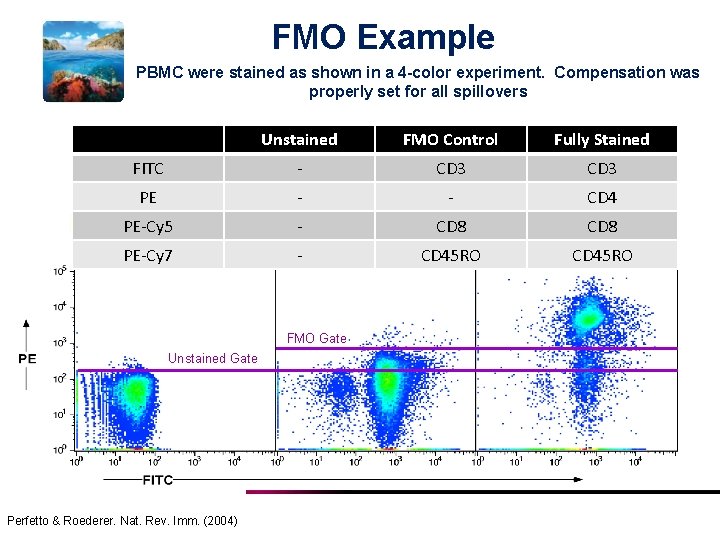
FMO Example PBMC were stained as shown in a 4 -color experiment. Compensation was properly set for all spillovers Unstained FMO Control Fully Stained FITC - CD 3 PE - - CD 4 PE-Cy 5 - CD 8 PE-Cy 7 - CD 45 RO FMO Gate Unstained Gate Perfetto & Roederer. Nat. Rev. Imm. (2004)

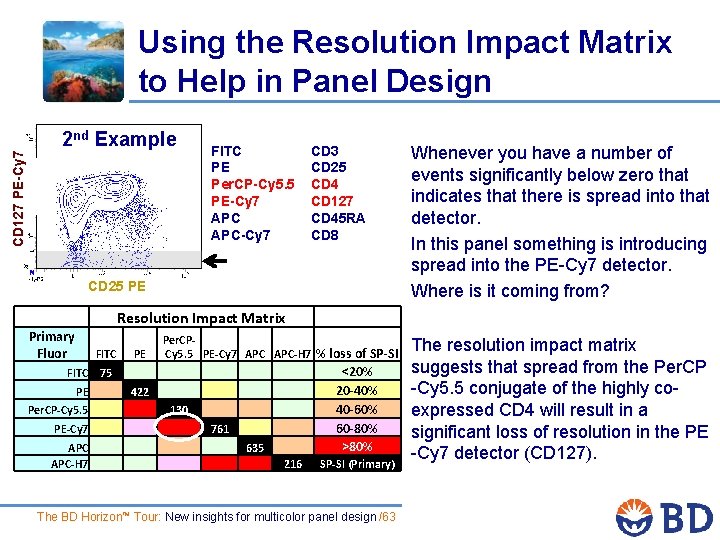
Using the Resolution Impact Matrix to Help in Panel Design 2 nd Example CD 127 PE-Cy 7 FITC PE Per. CP-Cy 5. 5 PE-Cy 7 APC-Cy 7 CD 3 CD 25 CD 4 CD 127 CD 45 RA CD 8 CD 25 PE Whenever you have a number of events significantly below zero that indicates that there is spread into that detector. In this panel something is introducing spread into the PE-Cy 7 detector. Where is it coming from? Resolution Impact Matrix Primary Fluor FITC PE Per. CP- Cy 5. 5 PE-Cy 7 APC-H 7 % loss of SP-SI PE 422 Per. CP-Cy 5. 5 130 PE-Cy 7 761 APC-H 7 635 <20% 20 -40% 40 -60% 60 -80% >80% 216 SP-SI (Primary) FITC 75 The BD Horizon™ Tour: New insights for multicolor panel design /63 The resolution impact matrix suggests that spread from the Per. CP -Cy 5. 5 conjugate of the highly coexpressed CD 4 will result in a significant loss of resolution in the PE -Cy 7 detector (CD 127).

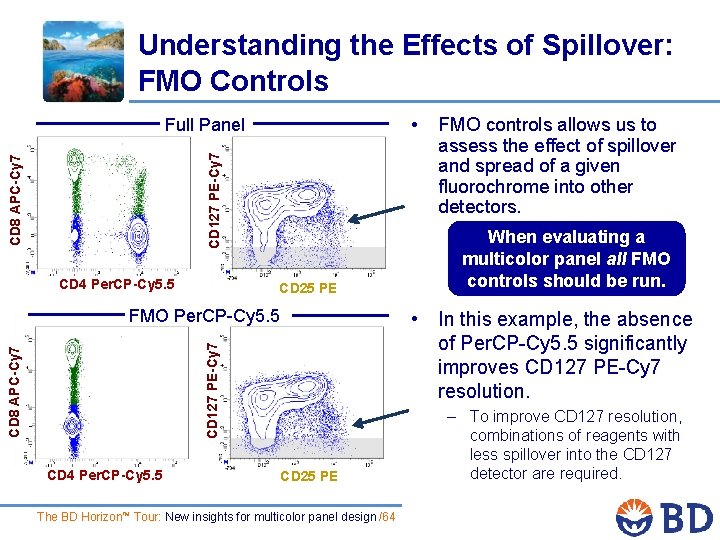
Understanding the Effects of Spillover: FMO Controls • CD 8 APC-Cy 7 CD 127 PE-Cy 7 Full Panel CD 4 Per. CP-Cy 5. 5 CD 25 PE FMO Per. CP-Cy 5. 5 When evaluating a multicolor panel all FMO controls should be run. • In this example, the absence of Per. CP-Cy 5. 5 significantly improves CD 127 PE-Cy 7 resolution. CD 127 PE-Cy 7 CD 8 APC-Cy 7 CD 4 Per. CP-Cy 5. 5 FMO controls allows us to assess the effect of spillover and spread of a given fluorochrome into other detectors. CD 25 PE The BD Horizon™ Tour: New insights for multicolor panel design /64 – To improve CD 127 resolution, combinations of reagents with less spillover into the CD 127 detector are required.

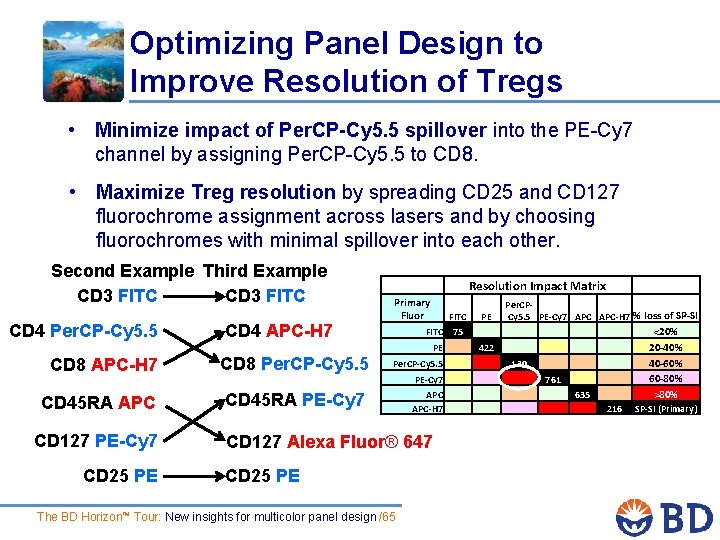
Optimizing Panel Design to Improve Resolution of Tregs • Minimize impact of Per. CP-Cy 5. 5 spillover into the PE-Cy 7 channel by assigning Per. CP-Cy 5. 5 to CD 8. • Maximize Treg resolution by spreading CD 25 and CD 127 fluorochrome assignment across lasers and by choosing fluorochromes with minimal spillover into each other. Second Example Third Example CD 3 FITC CD 4 Per. CP-Cy 5. 5 CD 4 APC-H 7 CD 8 Per. CP-Cy 5. 5 CD 45 RA APC CD 45 RA PE-Cy 7 CD 127 PE-Cy 7 CD 25 PE Resolution Impact Matrix Primary Fluor FITC PE Per. CP- Cy 5. 5 PE-Cy 7 APC-H 7 % loss of SP-SI PE 422 Per. CP-Cy 5. 5 130 PE-Cy 7 761 APC-H 7 635 <20% 20 -40% 40 -60% 60 -80% >80% 216 SP-SI (Primary) FITC 75 CD 127 Alexa Fluor® 647 CD 25 PE The BD Horizon™ Tour: New insights for multicolor panel design /65

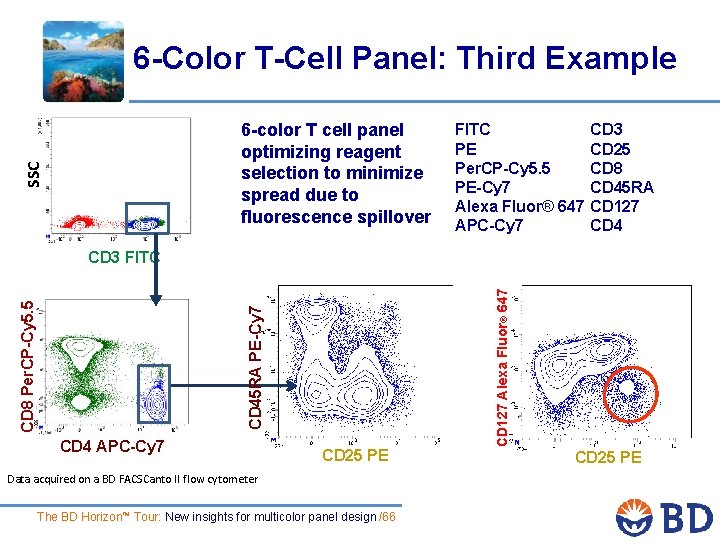
6 -Color T-Cell Panel: Third Example SSC 6 -color T cell panel optimizing reagent selection to minimize spread due to fluorescence spillover FITC PE Per. CP-Cy 5. 5 PE-Cy 7 Alexa Fluor® 647 APC-Cy 7 CD 3 CD 25 CD 8 CD 45 RA CD 127 CD 4 APC-Cy 7 CD 25 PE Data acquired on a BD FACSCanto II flow cytometer The BD Horizon™ Tour: New insights for multicolor panel design /66 CD 127 Alexa Fluor® 647 CD 45 RA PE-Cy 7 CD 8 Per. CP-Cy 5. 5 CD 3 FITC CD 25 PE

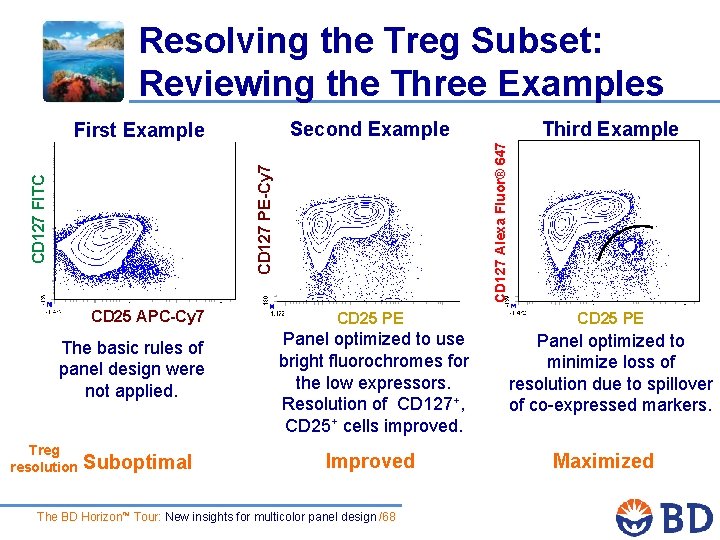
Resolving the Treg Subset: Reviewing the Three Examples Second Example CD 25 APC-Cy 7 The basic rules of panel design were not applied. Treg resolution Suboptimal Third Example CD 127 Alexa Fluor® 647 CD 127 FITC CD 127 PE-Cy 7 First Example CD 25 PE Panel optimized to use bright fluorochromes for the low expressors. Resolution of CD 127+, CD 25+ cells improved. Improved The BD Horizon™ Tour: New insights for multicolor panel design /68 CD 25 PE Panel optimized to minimize loss of resolution due to spillover of co-expressed markers. Maximized

Top Considerations What are the top three considerations for optimal panel design? – Fluorochrome brightness – Antigen density and co-expression – Spread due to spillover The BD Horizon™ Tour: New insights for multicolor panel design /69

High Parameter Considerations bdbiosciences. com /70 The BD Horizon™ Tour

COMPANY CONFIDENTIAL Maximizing resolution using instrument setup The goal of instrument setup for any application is to optimize the instrument to maximize the potential resolution Electronic Noise (Stain Index) of each detector. For each detector, find the gain setting (PMT voltage) that meets these two conditions: 350 V 450 V 1. Keeps (bright) positive cells on scale 2. Brings the negative population out of the Electronic Noise to maximize the resolution (Stain Index) 550 V

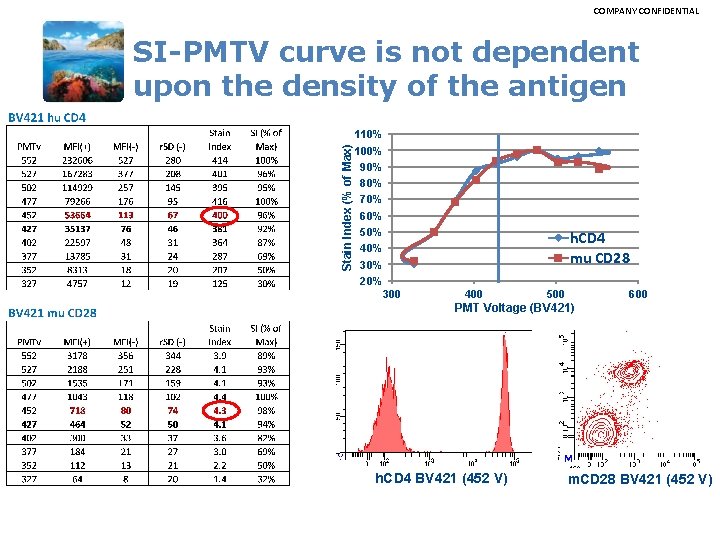
COMPANY CONFIDENTIAL Stain Index (% of Max) SI-PMTV curve is not dependent upon the density of the antigen 110% 100% 90% 80% 70% 60% 50% 40% 30% 20% 300 h. CD 4 mu CD 28 400 500 600 PMT Voltage (BV 421) h. CD 4 BV 421 (452 V) m. CD 28 BV 421 (452 V)

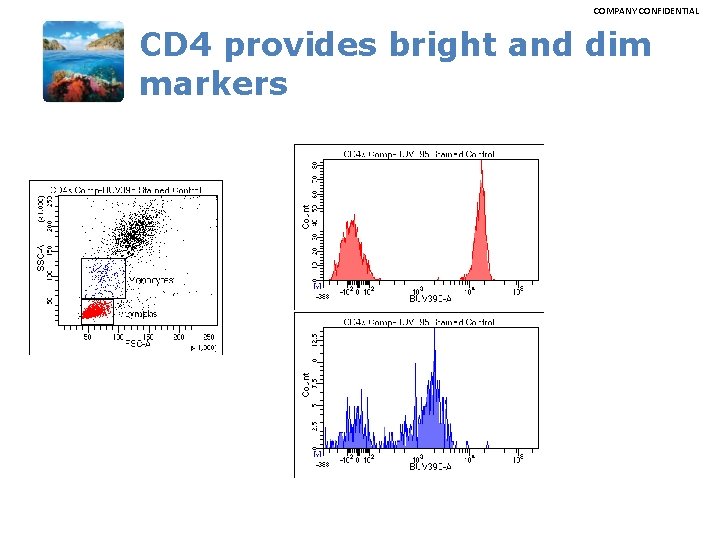
COMPANY CONFIDENTIAL CD 4 provides bright and dim markers

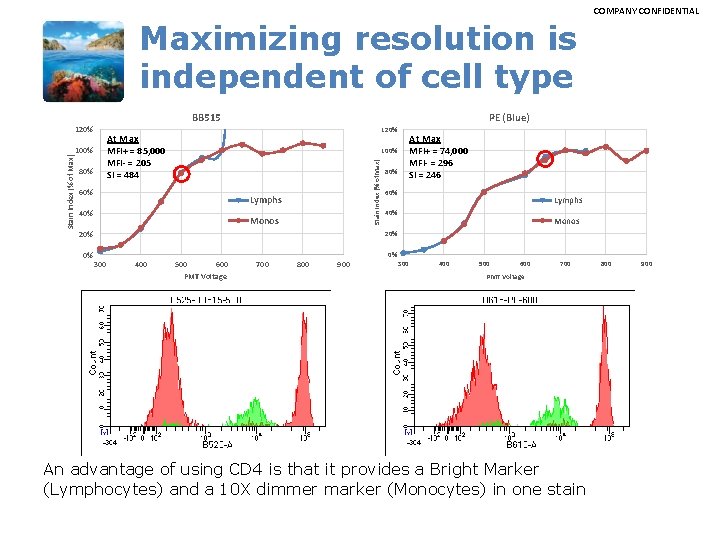
COMPANY CONFIDENTIAL Maximizing resolution is independent of cell type BB 515 PE (Blue) 120% At Max MFI+ = 85, 000 MFI- = 205 SI = 484 100% 80% At Max MFI+ = 74, 000 MFI- = 296 SI = 246 100% 60% Stain Index (% of Max) 120% Lymphs 40% Monos 20% 80% 60% Lymphs 40% Monos 20% 0% 0% 300 400 500 600 PMT Voltage 700 800 900 300 400 500 600 700 PMT Voltage An advantage of using CD 4 is that it provides a Bright Marker (Lymphocytes) and a 10 X dimmer marker (Monocytes) in one stain 800 900

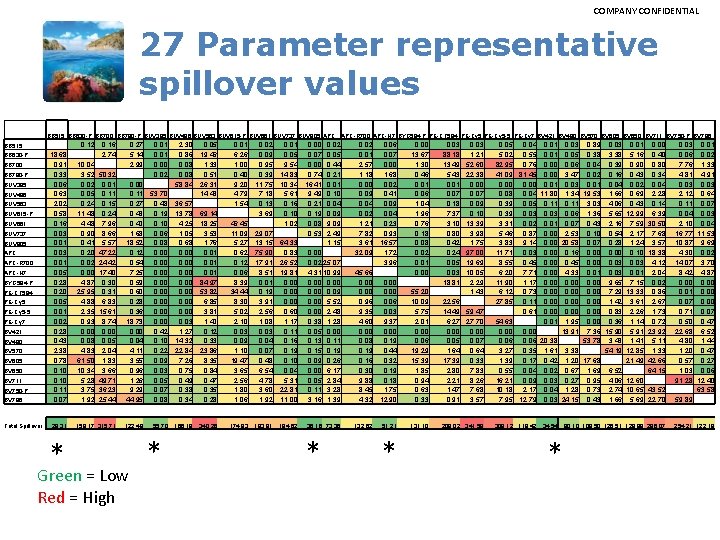
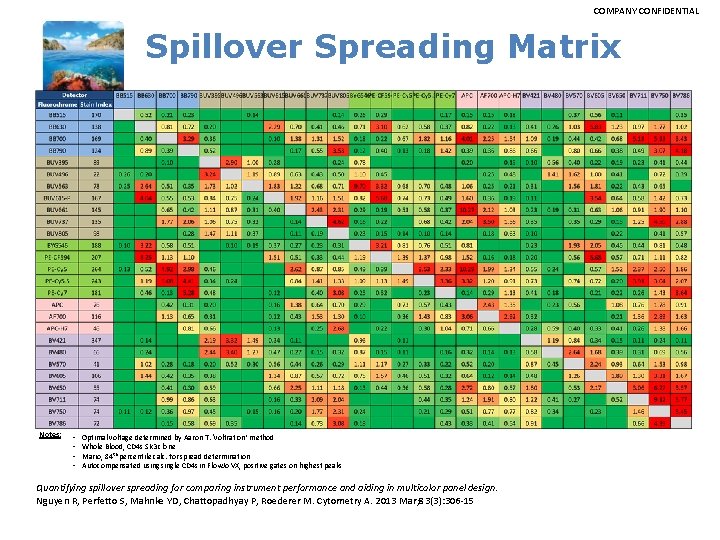
COMPANY CONFIDENTIAL 27 Parameter representative spillover values BB 515 BB 630 -P BB 700 BB 790 -P BUV 395 BUV 496 BUV 563 BUV 615 -P BUV 661 BUV 737 BUV 805 APC BB 515 0. 12 18. 68 0. 91 0. 33 0. 06 0. 63 2. 02 0. 58 0. 16 0. 03 0. 01 0. 05 0. 28 0. 20 0. 05 0. 01 0. 02 0. 28 0. 43 2. 38 0. 78 0. 10 0. 11 0. 07 BB 630 -P BB 700 BB 790 -P BUV 395 BUV 496 BUV 563 BUV 615 -P BUV 661 BUV 737 BUV 805 APC-R 700 APC-H 7 BYG 584 -P PE-CF 594 PE-Cy 5 -5 PE-Cy 7 BV 421 BV 480 BV 570 BV 605 BV 650 BV 711 BV 750 -P BV 786 Total Spillover 28. 31 * 0. 16 2. 74 0. 27 5. 14 2. 99 0. 01 0. 00 0. 02 2. 30 0. 86 0. 08 58. 84 0. 05 19. 46 1. 33 0. 51 26. 31 14. 43 0. 01 6. 26 1. 00 0. 40 9. 20 4. 79 1. 54 0. 02 0. 09 0. 95 0. 39 11. 75 7. 18 0. 13 3. 69 0. 01 0. 05 9. 54 14. 83 10. 34 5. 61 0. 16 0. 10 1. 02 0. 00 0. 07 0. 00 0. 74 16. 41 9. 49 0. 21 0. 19 0. 08 0. 53 APC-R 700 APC-H 7 BYG 584 -P PE-CF 594 PE-Cy 5 -5 PE-Cy 7 BV 421 BV 480 BV 570 BV 605 BV 650 BV 711 BV 750 -P BV 786 0. 02 0. 05 0. 44 0. 21 0. 01 0. 10 0. 04 0. 09 9. 09 2. 49 1. 15 10. 04 3. 52 50. 32 0. 01 0. 05 0. 11 0. 24 0. 15 11. 48 0. 24 4. 48 7. 96 0. 90 8. 66 0. 41 5. 57 0. 20 47. 22 0. 02 24. 42 0. 00 17. 40 4. 87 0. 30 25. 95 0. 31 4. 88 6. 83 2. 35 15. 61 0. 93 8. 74 0. 00 0. 08 0. 05 4. 83 2. 04 61. 50 1. 83 10. 34 3. 66 5. 28 49. 71 3. 75 36. 23 1. 92 25. 44 0. 00 0. 11 53. 70 0. 27 0. 48 36. 57 0. 48 0. 19 13. 78 69. 14 0. 40 0. 10 4. 25 18. 25 1. 68 0. 06 1. 05 3. 53 18. 52 0. 08 0. 68 1. 76 0. 12 0. 00 0. 01 0. 54 0. 00 0. 01 7. 25 0. 00 0. 01 0. 59 0. 00 84. 97 0. 60 0. 00 53. 82 0. 28 0. 00 6. 85 0. 36 0. 00 3. 81 18. 73 0. 00 0. 03 1. 40 0. 00 0. 42 1. 27 0. 12 0. 04 0. 10 14. 32 0. 33 4. 11 0. 22 22. 84 23. 86 3. 55 0. 09 7. 26 8. 35 0. 96 0. 03 0. 75 0. 84 1. 26 0. 05 0. 49 0. 47 9. 29 0. 07 0. 38 0. 35 44. 95 0. 08 0. 34 0. 28 46. 45 11. 09 5. 27 0. 62 0. 12 0. 06 8. 39 34. 44 8. 30 5. 02 2. 10 0. 03 0. 09 1. 10 19. 47 3. 65 2. 56 1. 80 1. 06 29. 07 13. 15 75. 90 17. 91 8. 51 0. 01 0. 19 3. 91 2. 56 1. 08 0. 03 0. 04 0. 07 0. 48 6. 54 4. 78 3. 60 1. 92 64. 33 0. 83 26. 52 19. 81 0. 00 0. 60 1. 17 0. 11 0. 16 0. 19 0. 10 0. 04 5. 31 22. 81 11. 00 0. 02 25. 07 4. 31 10. 99 0. 00 0. 09 0. 00 5. 52 0. 00 2. 48 0. 38 1. 28 0. 05 0. 00 0. 13 0. 11 0. 15 0. 19 0. 09 0. 26 0. 00 6. 17 0. 05 2. 84 0. 11 3. 28 3. 16 1. 39 158. 17 315. 71 122. 49 174. 83 193. 91 194. 62 36. 16 73. 36 Green = Low Red = High 55. 70 * 166. 18 340. 26 * 0. 02 0. 01 2. 57 1. 18 0. 00 0. 09 0. 04 0. 02 1. 21 7. 82 3. 61 32. 09 0. 06 0. 07 0. 00 1. 68 0. 02 0. 41 0. 09 0. 04 0. 23 0. 93 16. 57 1. 72 3. 96 45. 66 0. 00 0. 96 0. 06 9. 35 0. 03 4. 60 9. 37 0. 00 0. 08 0. 19 0. 44 0. 16 0. 32 0. 30 0. 19 9. 88 0. 18 8. 45 1. 75 4. 32 12. 90 132. 62 51. 21 * 0. 00 13. 67 1. 30 0. 46 0. 01 0. 06 1. 04 1. 96 0. 76 0. 18 0. 02 0. 01 0. 00 0. 03 88. 18 13. 49 5. 43 0. 01 0. 07 0. 18 7. 37 3. 10 0. 80 0. 42 0. 24 0. 05 0. 03 18. 81 0. 03 1. 21 52. 60 22. 38 0. 00 0. 07 0. 09 0. 10 13. 39 3. 98 1. 75 97. 00 19. 69 10. 05 2. 29 1. 43 55. 20 10. 09 5. 75 2. 01 0. 00 0. 06 19. 29 15. 39 1. 85 0. 94 0. 63 0. 33 22. 56 14. 49 59. 47 6. 27 27. 70 0. 00 0. 05 0. 07 1. 64 0. 64 17. 39 0. 33 2. 80 7. 83 2. 21 8. 26 1. 47 7. 68 0. 91 3. 57 0. 05 0. 04 0. 01 0. 03 0. 89 0. 03 0. 01 0. 00 5. 02 0. 55 0. 01 0. 05 0. 38 3. 38 5. 16 0. 40 82. 95 0. 76 0. 00 0. 06 0. 04 0. 39 0. 90 0. 80 41. 09 81. 45 0. 00 3. 47 0. 02 0. 16 0. 43 0. 34 0. 00 0. 01 0. 03 0. 01 0. 04 0. 02 0. 04 0. 08 0. 04 11. 80 1. 34 19. 53 1. 66 0. 69 2. 28 0. 39 0. 05 0. 11 3. 03 4. 06 0. 43 0. 14 0. 39 0. 03 0. 06 1. 36 5. 65 12. 99 6. 39 3. 31 0. 02 0. 01 0. 07 0. 43 2. 16 7. 59 30. 50 5. 46 0. 87 0. 00 2. 53 0. 10 0. 54 2. 17 7. 68 3. 83 9. 14 0. 00 20. 58 0. 07 0. 28 1. 24 3. 57 11. 71 0. 03 0. 00 0. 16 0. 00 0. 10 18. 38 8. 55 0. 45 0. 00 0. 03 4. 12 6. 20 7. 71 0. 00 4. 33 0. 01 0. 03 0. 01 2. 04 11. 90 1. 17 0. 00 9. 65 7. 15 0. 02 6. 12 0. 73 0. 00 7. 29 13. 33 0. 86 27. 85 0. 11 0. 00 1. 42 3. 61 2. 67 0. 61 0. 00 0. 83 2. 26 1. 73 54. 63 0. 01 1. 95 0. 00 0. 36 1. 14 0. 72 0. 00 13. 91 7. 36 15. 90 5. 91 23. 92 0. 06 20. 38 53. 78 3. 48 1. 41 5. 11 3. 27 0. 35 1. 61 3. 38 54. 19 12. 85 1. 33 1. 39 0. 17 0. 42 1. 20 17. 68 21. 49 42. 66 0. 55 0. 04 0. 02 0. 67 1. 69 6. 52 64. 15 16. 21 0. 09 0. 03 0. 27 0. 95 4. 06 12. 60 10. 18 2. 17 0. 04 1. 28 0. 73 2. 74 10. 65 43. 52 7. 95 12. 79 0. 03 24. 15 0. 43 1. 66 5. 69 22. 70 131. 10 208. 02 341. 59 309. 12 119. 42 34. 54 * 80. 10 108. 50 126. 51 129. 88 286. 07 0. 03 0. 01 0. 06 0. 02 7. 76 1. 33 4. 81 4. 91 0. 03 2. 12 0. 64 0. 11 0. 07 0. 04 0. 03 2. 10 0. 04 16. 77 11. 53 10. 87 9. 69 4. 30 0. 02 14. 07 3. 70 8. 42 4. 87 0. 00 0. 01 0. 00 0. 07 0. 00 0. 71 0. 07 0. 50 0. 47 22. 68 6. 52 4. 80 1. 44 1. 20 0. 47 0. 57 0. 27 1. 03 0. 06 91. 28 12. 40 63. 58 59. 89 254. 21 122. 18

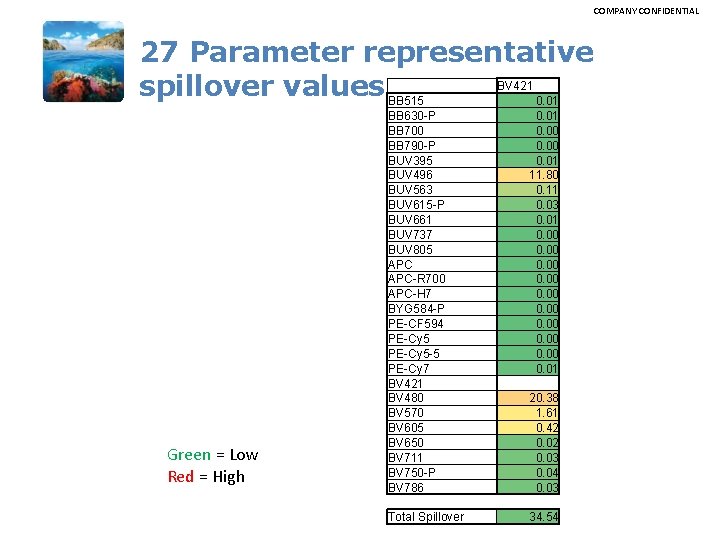
COMPANY CONFIDENTIAL 27 Parameter representative BV 421 spillover values BB 515 0. 01 Green = Low Red = High BB 630 -P BB 700 BB 790 -P BUV 395 BUV 496 BUV 563 BUV 615 -P BUV 661 BUV 737 BUV 805 APC-R 700 APC-H 7 BYG 584 -P PE-CF 594 PE-Cy 5 -5 PE-Cy 7 BV 421 BV 480 BV 570 BV 605 BV 650 BV 711 BV 750 -P BV 786 Total Spillover 0. 01 0. 00 0. 01 11. 80 0. 11 0. 03 0. 01 0. 00 0. 01 20. 38 1. 61 0. 42 0. 03 0. 04 0. 03 34. 54

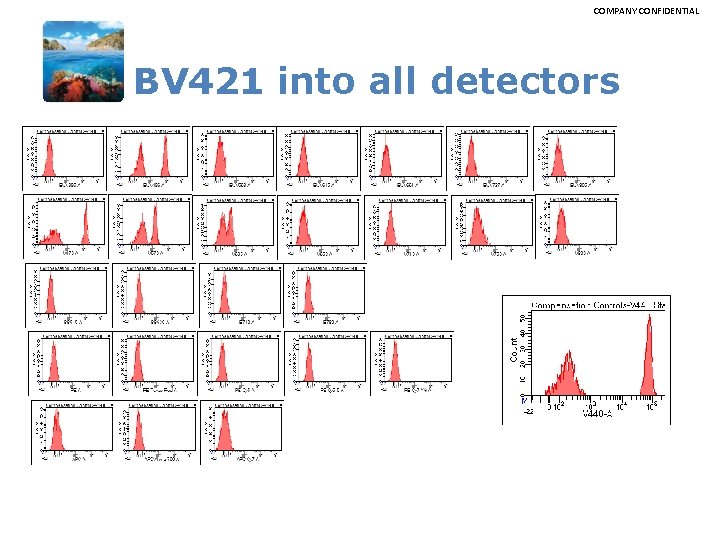
COMPANY CONFIDENTIAL BV 421 into all detectors

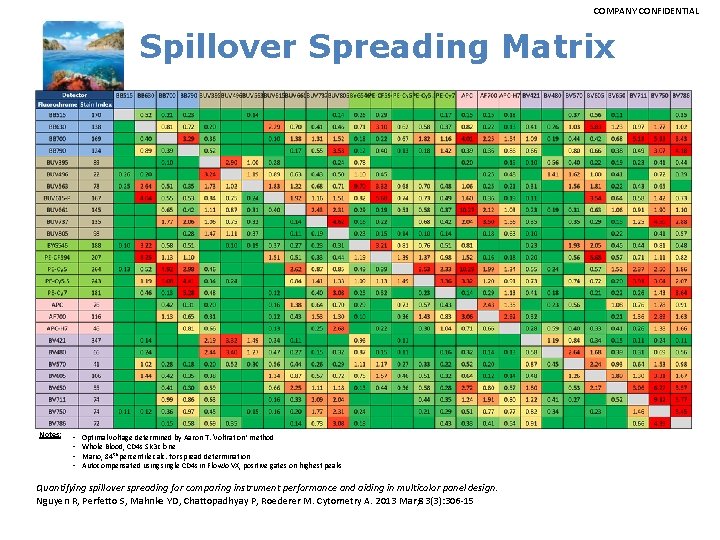
COMPANY CONFIDENTIAL Spillover Spreading Matrix Notes: • • Optimal voltage determined by Aaron T. ‘voltration’ method Whole Blood, CD 4 s SK 3 clone Mario, 84 th percentile calc. for spread determination Autocompensated usingle CD 4 s in Flow. Jo VX, positive gates on highest peaks Quantifying spillover spreading for comparing instrument performance and aiding in multicolor panel design. Nguyen R, Perfetto S, Mahnke YD, Chattopadhyay P, Roederer M. Cytometry A. 2013 Mar; 83(3): 306 -15

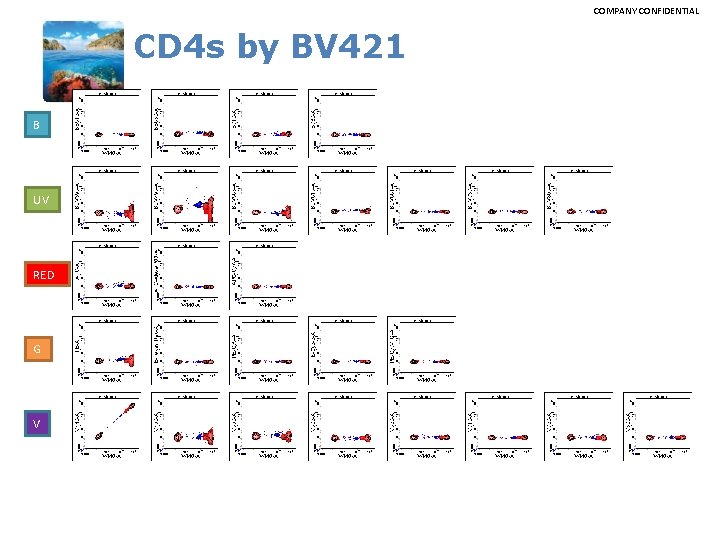
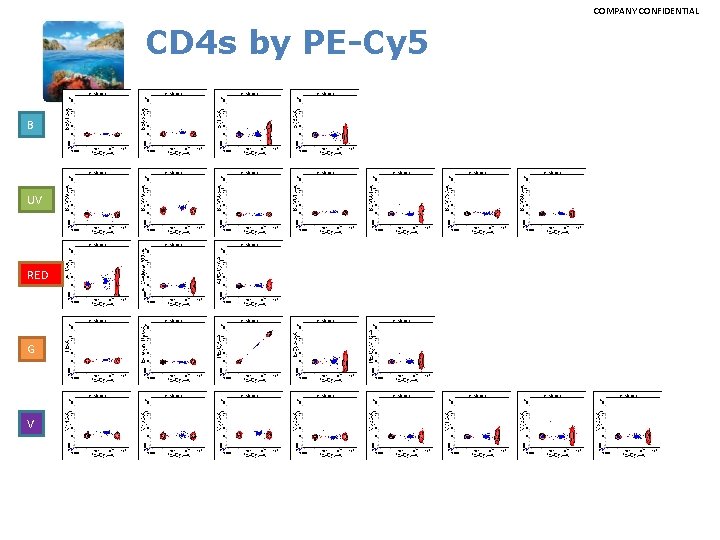
COMPANY CONFIDENTIAL CD 4 s by BV 421 B UV RED G V

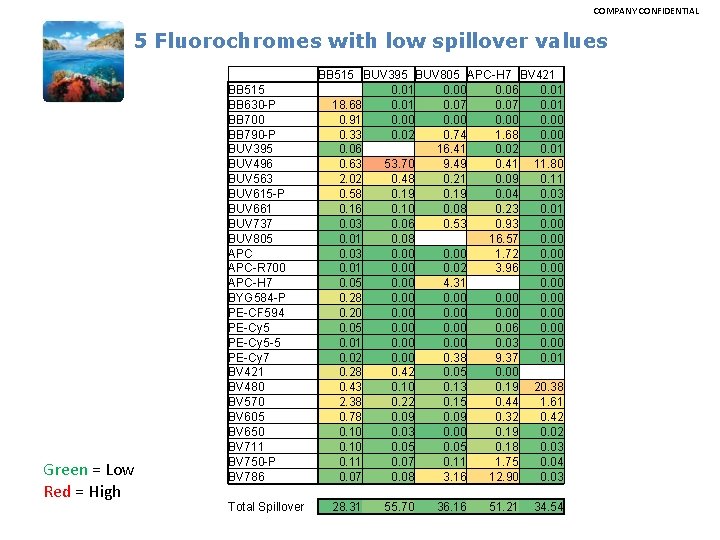
COMPANY CONFIDENTIAL 5 Fluorochromes with low spillover values Green = Low Red = High BB 515 BB 630 -P BB 700 BB 790 -P BUV 395 BUV 496 BUV 563 BUV 615 -P BUV 661 BUV 737 BUV 805 APC-R 700 APC-H 7 BYG 584 -P PE-CF 594 PE-Cy 5 -5 PE-Cy 7 BV 421 BV 480 BV 570 BV 605 BV 650 BV 711 BV 750 -P BV 786 Total Spillover BB 515 BUV 395 BUV 805 APC-H 7 BV 421 0. 01 0. 00 0. 06 0. 01 18. 68 0. 01 0. 07 0. 01 0. 91 0. 00 0. 33 0. 02 0. 74 1. 68 0. 00 0. 06 16. 41 0. 02 0. 01 0. 63 53. 70 9. 49 0. 41 11. 80 2. 02 0. 48 0. 21 0. 09 0. 11 0. 58 0. 19 0. 04 0. 03 0. 16 0. 10 0. 08 0. 23 0. 01 0. 03 0. 06 0. 53 0. 93 0. 00 0. 01 0. 08 16. 57 0. 00 0. 03 0. 00 1. 72 0. 00 0. 01 0. 00 0. 02 3. 96 0. 00 0. 05 0. 00 4. 31 0. 00 0. 28 0. 00 0. 20 0. 00 0. 05 0. 00 0. 06 0. 00 0. 01 0. 00 0. 03 0. 00 0. 02 0. 00 0. 38 9. 37 0. 01 0. 28 0. 42 0. 05 0. 00 0. 43 0. 10 0. 13 0. 19 20. 38 2. 38 0. 22 0. 15 0. 44 1. 61 0. 78 0. 09 0. 32 0. 42 0. 10 0. 03 0. 00 0. 19 0. 02 0. 10 0. 05 0. 18 0. 03 0. 11 0. 07 0. 11 1. 75 0. 04 0. 07 0. 08 3. 16 12. 90 0. 03 28. 31 55. 70 36. 16 51. 21 34. 54

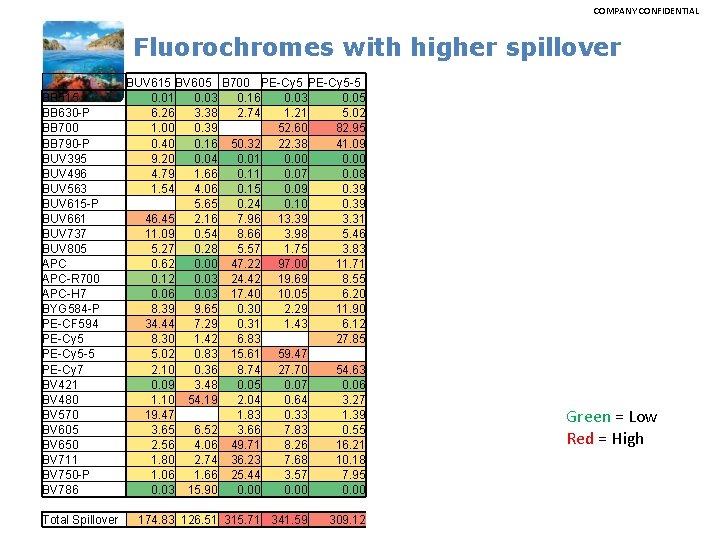
COMPANY CONFIDENTIAL Fluorochromes with higher spillover BB 515 BB 630 -P BB 700 BB 790 -P BUV 395 BUV 496 BUV 563 BUV 615 -P BUV 661 BUV 737 BUV 805 APC-R 700 APC-H 7 BYG 584 -P PE-CF 594 PE-Cy 5 -5 PE-Cy 7 BV 421 BV 480 BV 570 BV 605 BV 650 BV 711 BV 750 -P BV 786 Total Spillover BUV 615 BV 605 B 700 PE-Cy 5 -5 0. 01 0. 03 0. 16 0. 03 0. 05 6. 26 3. 38 2. 74 1. 21 5. 02 1. 00 0. 39 52. 60 82. 95 0. 40 0. 16 50. 32 22. 38 41. 09 9. 20 0. 04 0. 01 0. 00 4. 79 1. 66 0. 11 0. 07 0. 08 1. 54 4. 06 0. 15 0. 09 0. 39 5. 65 0. 24 0. 10 0. 39 46. 45 2. 16 7. 96 13. 39 3. 31 11. 09 0. 54 8. 66 3. 98 5. 46 5. 27 0. 28 5. 57 1. 75 3. 83 0. 62 0. 00 47. 22 97. 00 11. 71 0. 12 0. 03 24. 42 19. 69 8. 55 0. 06 0. 03 17. 40 10. 05 6. 20 8. 39 9. 65 0. 30 2. 29 11. 90 34. 44 7. 29 0. 31 1. 43 6. 12 8. 30 1. 42 6. 83 27. 85 5. 02 0. 83 15. 61 59. 47 2. 10 0. 36 8. 74 27. 70 54. 63 0. 09 3. 48 0. 05 0. 07 0. 06 1. 10 54. 19 2. 04 0. 64 3. 27 19. 47 1. 83 0. 33 1. 39 3. 65 6. 52 3. 66 7. 83 0. 55 2. 56 4. 06 49. 71 8. 26 16. 21 1. 80 2. 74 36. 23 7. 68 10. 18 1. 06 1. 66 25. 44 3. 57 7. 95 0. 03 15. 90 0. 00 174. 83 126. 51 315. 71 341. 59 309. 12 Green = Low Red = High

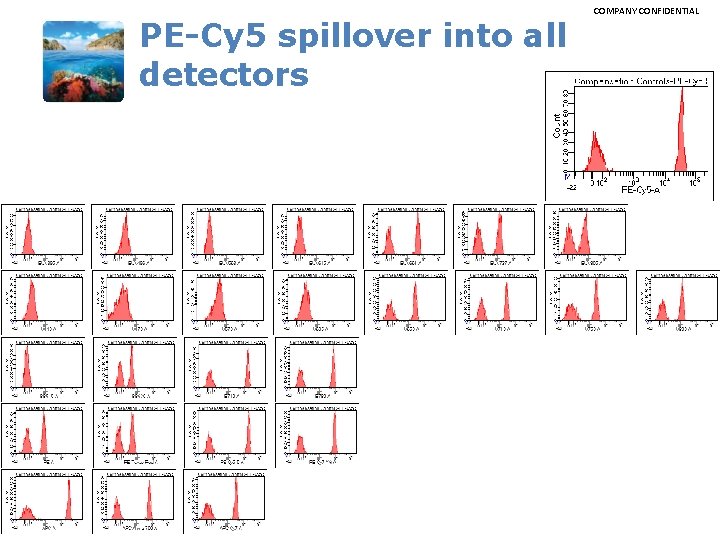
PE-Cy 5 spillover into all detectors COMPANY CONFIDENTIAL

COMPANY CONFIDENTIAL Spillover Spreading Matrix Notes: • • Optimal voltage determined by Aaron T. ‘voltration’ method Whole Blood, CD 4 s SK 3 clone Mario, 84 th percentile calc. for spread determination Autocompensated usingle CD 4 s in Flow. Jo VX, positive gates on highest peaks Quantifying spillover spreading for comparing instrument performance and aiding in multicolor panel design. Nguyen R, Perfetto S, Mahnke YD, Chattopadhyay P, Roederer M. Cytometry A. 2013 Mar; 83(3): 306 -15

COMPANY CONFIDENTIAL CD 4 s by PE-Cy 5 B UV RED G V

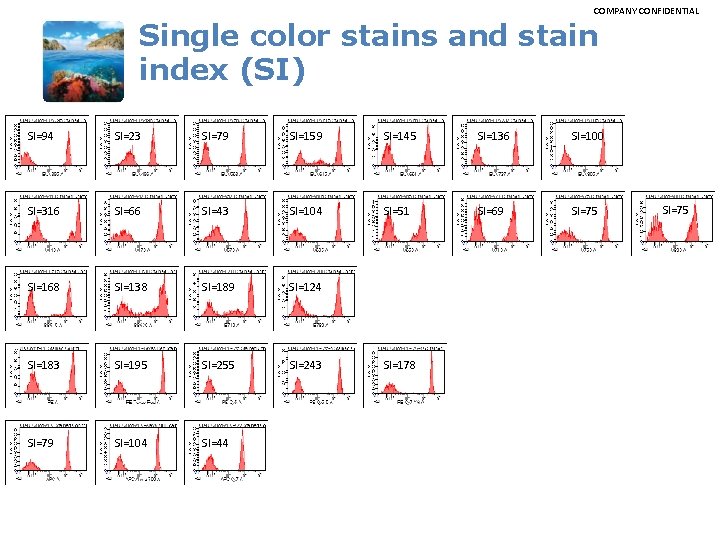
COMPANY CONFIDENTIAL Single color stains and stain index (SI) SI=94 SI=23 SI=79 SI=159 SI=145 SI=136 SI=100 SI=316 SI=66 SI=43 SI=104 SI=51 SI=69 SI=75 SI=168 SI=138 SI=189 SI=124 SI=183 SI=195 SI=255 SI=243 SI=79 SI=104 SI=44 SI=178 SI=75

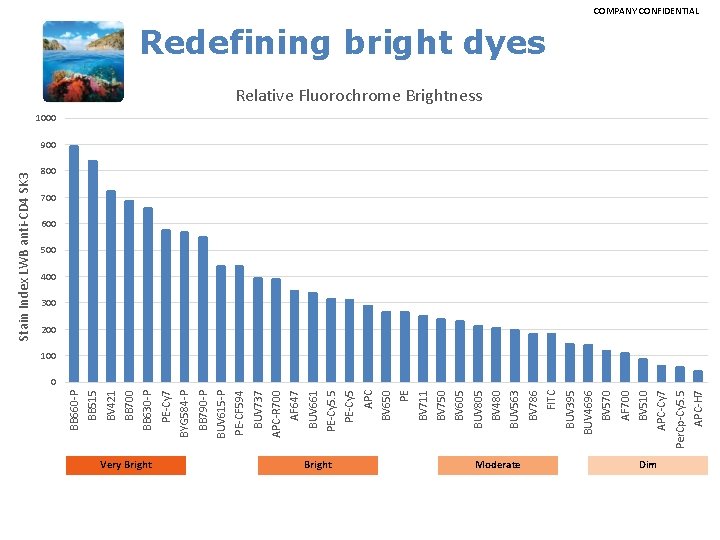
Very Bright Moderate Dim APC-H 7 Per. Cp-Cy 5. 5 APC-Cy 7 BV 510 AF 700 BV 570 BUV 4696 BUV 395 FITC BV 786 BUV 563 BV 480 BUV 805 BV 605 BV 750 BV 711 PE BV 650 APC PE-Cy 5. 5 BUV 661 AF 647 APC-R 700 BUV 737 PE-CF 594 BUV 615 -P BB 790 -P BYG 584 -P PE-Cy 7 BB 630 -P BB 700 BV 421 BB 515 BB 660 -P Stain Index LWB anti-CD 4 SK 3 COMPANY CONFIDENTIAL Redefining bright dyes Relative Fluorochrome Brightness 1000 900 800 700 600 500 400 300 200 100 0

COMPANY CONFIDENTIAL 20+ Color broad immunophenotyping panel Acquire biologically stained samples at Operational Voltages • Power up instrument • Stain samples • Set up experiment • Acquire all samples • Quick analysis for verification of panel staining • Review analysis of previously acquired sample Supporting Documents • 20+C Staining and Acquisition Protocol • Panel Staining Document

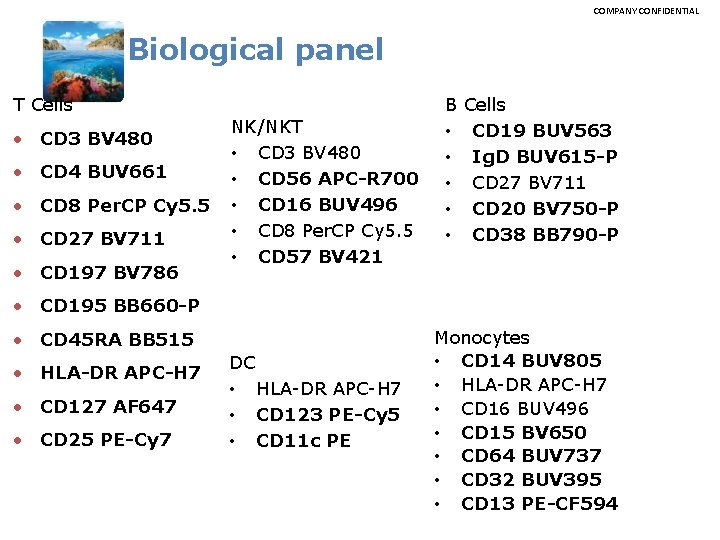
COMPANY CONFIDENTIAL Biological panel T Cells • CD 3 BV 480 • CD 4 BUV 661 • CD 8 Per. CP Cy 5. 5 • CD 27 BV 711 • CD 197 BV 786 NK/NKT • CD 3 BV 480 • CD 56 APC-R 700 • CD 16 BUV 496 • CD 8 Per. CP Cy 5. 5 • CD 57 BV 421 B • • • Cells CD 19 BUV 563 Ig. D BUV 615 -P CD 27 BV 711 CD 20 BV 750 -P CD 38 BB 790 -P • CD 195 BB 660 -P • CD 45 RA BB 515 • HLA-DR APC-H 7 • CD 127 AF 647 • CD 25 PE-Cy 7 DC • HLA-DR APC-H 7 • CD 123 PE-Cy 5 • CD 11 c PE Monocytes • CD 14 BUV 805 • HLA-DR APC-H 7 • CD 16 BUV 496 • CD 15 BV 650 • CD 64 BUV 737 • CD 32 BUV 395 • CD 13 PE-CF 594

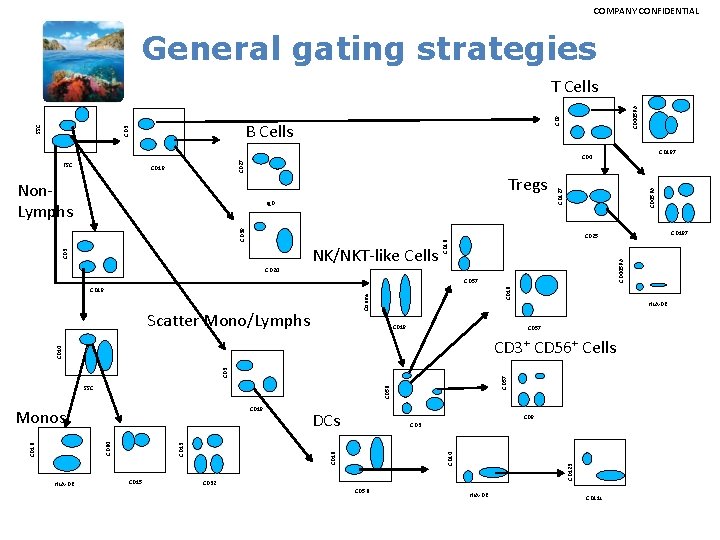
COMPANY CONFIDENTIAL General gating strategies CD 38 Ig. D CD 445 RA CD 45 RA Tregs Non. Lymphs CD 127 CD 19 CD 445 RA CD 16 CD 3 NK/NKT-like Cells Counts CD 16 CD 57 CD 19 Scatter Mono/Lymphs CD 19 CD 57 CD 56 CD 57 CD 3 CD 14 CD 8 CD 32 CD 14 CD 16 CD 13 CD 15 DCs CD 56 CD 123 CD 19 CD 64 CD 16 HLA-DR CD 3+ CD 56+ Cells SSC HLA-DR CD 197 CD 25 CD 20 Monos CD 197 CD 4 CD 27 FSC CD 8 B Cells CD 3 SSC T Cells HLA-DR CD 11 c

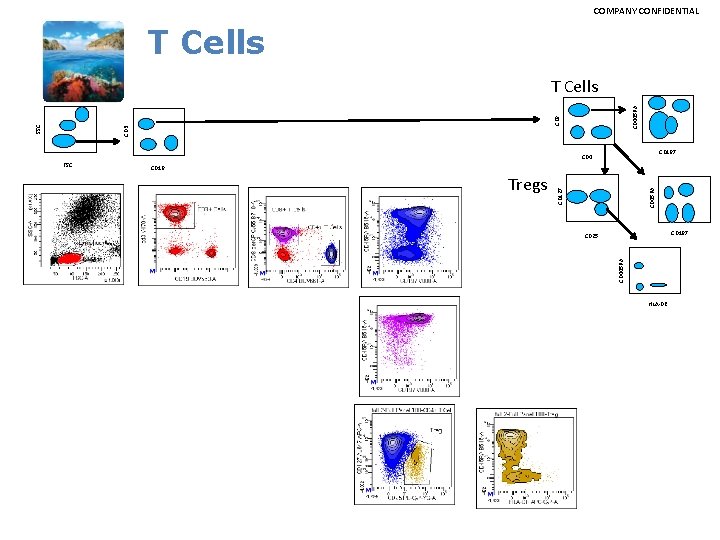
COMPANY CONFIDENTIAL T Cells CD 3 SSC CD 8 CD 445 RA T Cells CD 197 CD 4 CD 197 CD 25 CD 445 RA Tregs CD 45 RA CD 19 CD 127 FSC HLA-DR

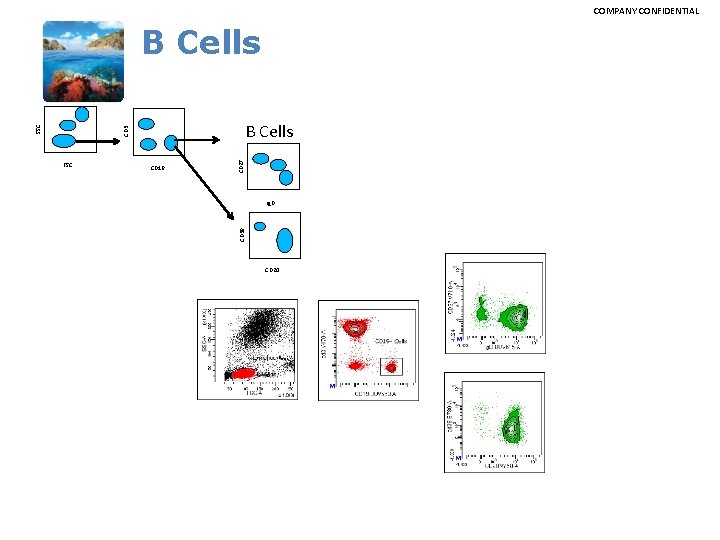
COMPANY CONFIDENTIAL B Cells CD 19 Ig. D CD 38 FSC CD 27 CD 3 SSC B Cells CD 20

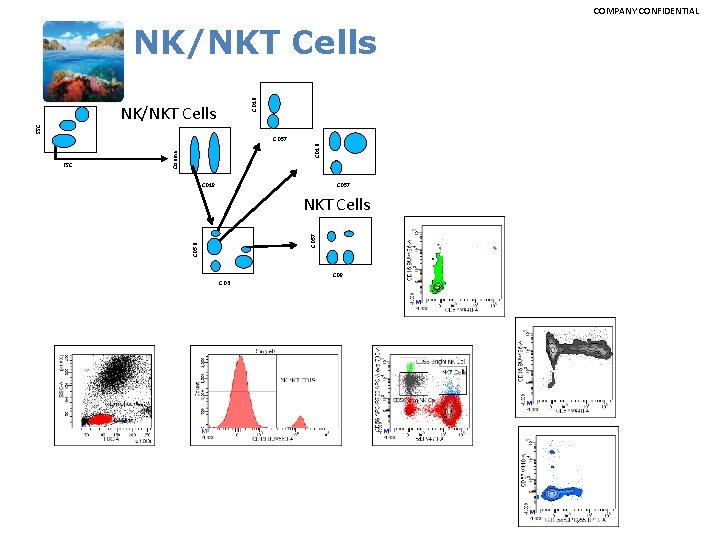
COMPANY CONFIDENTIAL CD 16 NK/NKT Cells SSC NK/NKT Cells Counts CD 19 CD 57 NKT Cells CD 56 FSC CD 16 CD 57 CD 8 CD 3

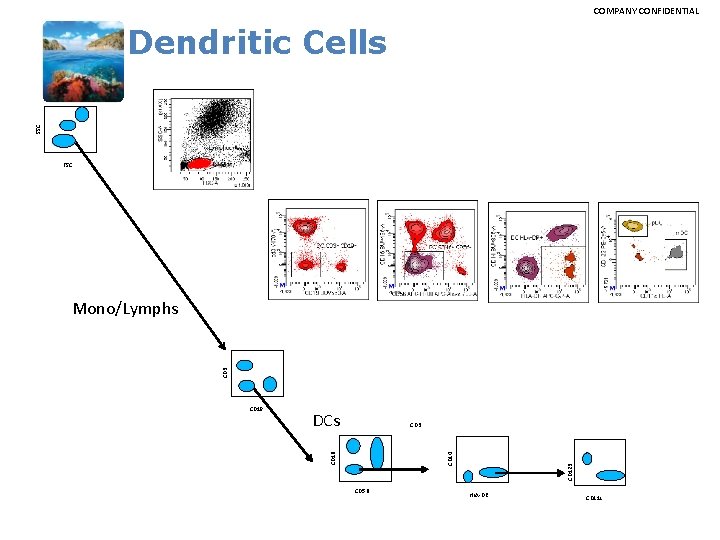
COMPANY CONFIDENTIAL SSC Dendritic Cells FSC CD 3 Mono/Lymphs DCs CD 3 CD 56 CD 123 CD 14 CD 16 CD 19 HLA-DR CD 11 c

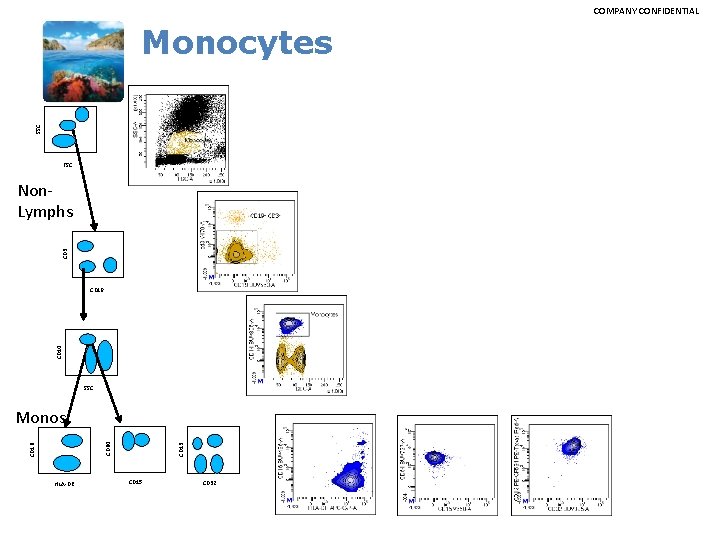
COMPANY CONFIDENTIAL SSC Monocytes FSC CD 3 Non. Lymphs CD 14 CD 19 SSC HLA-DR CD 13 CD 16 CD 64 Monos CD 15 CD 32

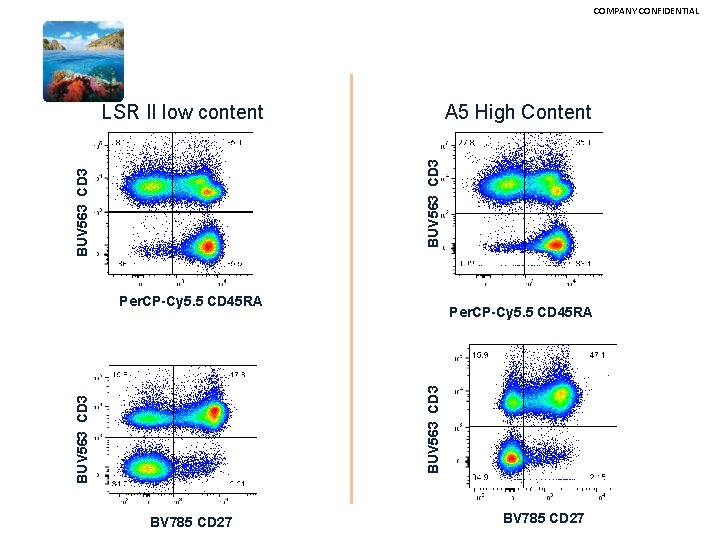
COMPANY CONFIDENTIAL Comparing established vs new technology 17 Color LSRII vs Symphony A 5

COMPANY CONFIDENTIAL Comparison New dye constructs were compared to a low content LSRII configuration to a High Content X 50. The laser powers were set to same level BD FACSymphony A 5 • • • Violet laser – 10 color Decagon UV Laser detection – 10 color Decagon Blue Laser detection – 6 color Polygon Red Laser detection – 3 color Polygon YG Laser detection – 8 color Polygon 17 Color LSRII • • • Violet laser – 6 color Octagon UV Laser detection – 2 color Trigon Blue Laser detection – 2 color Trigon Red Laser detection – 3 color Trigon YG Laser detection – 3 color Trigon • • • • • BUV 395 CD 4 BUV 563 CD 3 BUV 805 CD 8 BV 421 CXCR 5 BV 510 Ig. D BV 605 Va 7. 2 BV 650 CD 94 BV 711 CD 19 BV 785 CD 27 BB 515 CD 25 Per. CP-Cy 5. 5 CD 45 RA PE CCR 7 PE-CF 594 CD 28 PE-Cy 7 CCR 4 APC CD 127 Alexa. Fluor 700 CD 45 Live_or_dye 750/777

COMPANY CONFIDENTIAL A 5 High Content BUV 563 CD 3 LSR II low content Per. CP-Cy 5. 5 CD 45 RA BUV 563 CD 3 Per. CP-Cy 5. 5 CD 45 RA BV 785 CD 27

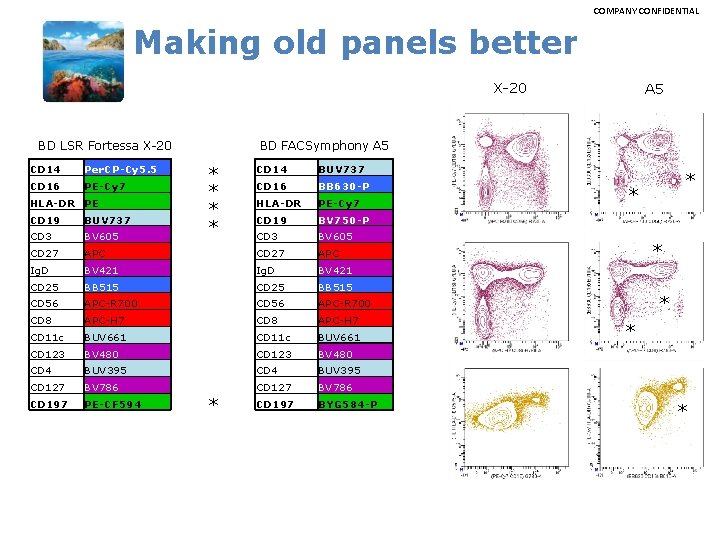
COMPANY CONFIDENTIAL Making old panels better X-20 BD FACSymphony A 5 BD LSR Fortessa X-20 CD 14 Per. CP-Cy 5. 5 CD 16 PE-Cy 7 HLA-DR PE A 5 * * CD 14 BUV 737 CD 16 BB 630 -P HLA-DR PE-Cy 7 CD 19 BV 750 -P CD 3 BV 605 CD 19 BUV 737 CD 3 BV 605 CD 27 APC Ig. D BV 421 CD 25 BB 515 CD 56 APC-R 700 CD 8 APC-H 7 CD 11 c BUV 661 CD 123 BV 480 CD 4 BUV 395 CD 127 BV 786 CD 197 PE-CF 594 CD 197 BYG 584 -P * * * *

Thank you Tools: www. bdbiosciences. com/tools • Detailed fluorochrome information • Buffer compatibility resource • Multicolor panel designer • Spectrum viewer The BD Horizon™ Tour: New insights for multicolor panel design /99 Resources: researchapplications@bd. com anthony. steichen@bd. com 877. 232. 8995, 3, 2