MULLERROCHOW PROCESS AND CURING OF SILICONE RUBBERS Presented

MULLER-ROCHOW PROCESS AND CURING OF SILICONE RUBBERS Presented by DR. MUKESH KUMAR ASSISTANT PROFESSOR Department of Chemistry Vaish College, Rohtak For B. Sc 6 nd sem students
MULLER-ROCHOW PROCESS The Rochow process is the most commercially important reaction in the preparation of silicone compounds. In it methyl chloride is reacted with solid silicon metal, in the presence of copper catalysts and certain promoters to produce a mixture of chlorosilanes. Simplistically, the overall reaction is as follows: 300 o C 2 CH 3 -Cl + Si --------> Me 2 Si. Cl 2 Catalyst However, a complex mixture of products is actually achieved. Rochow Process (SYNTHESIS OF CHLOROSILANES) Si + 2 CH 3 Cl -----> (CH 3)2 -Si-Cl 2 (Predominate) (CH 3)3 -Si-Cl CH 3 -Si-Cl 3 Si-Cl 4 CH 3 HSi. Cl 2 (CH 3)2 HSi. Cl Others The predominant material obtained is dimethyldichlorosilane (approx. 80% by weight).

MULLER-ROCHOW PROCESS The next most abundant compounds in order of concentration are methyltrichlorosilane, followed by trimethylchlorosilane and methylhydrogendichlorosilane. This composition information is very important since it drives the economics of the silicone business. Since every pound of chlorosilanes produced results in the distribution of products described, one must balance allocation of costs of each material in proportion to the amount produced and the demand for each. In order to operate this business profitably one must sell every pound of product produced. This by definition makes this a commodity business. Specialty producers on the other hand make what they can sell and do not have to balance by-product and co-product streams.
MULLER-ROCHOW PROCESS Since many silicone surfactants are based upon methylhydrogendichlorosilane, a relatively minor component of the silane stream, the cost of these materials is high, relative to silicone fluids, based upon dimethyldichlorosilane. The reaction to make chlorosilanes is quite complex and is carried out at a temperature of about 300 o C, under pressures typically of 3 bars. The reaction mass needs to be heated in order to obtain reaction, but once the reaction temperature is reached, the reaction becomes exothermic, and consequently requires very stringent temperature control. The reaction is carried out in a fluidized bed reactor and occurs in a solid / gaseous reaction. In order to maximize the reaction efficiency, the solid silicon must be low in other metallic components.
MULLER-ROCHOW PROCESS The fine residue that is extracted from the process is dependant upon the quality of the silicon going into the process but is generally made up of Cu, Fe, Al, and Ca. Consequently, silicon having low concentrations of these elements is desired for the process.

Silicone rubber. Silicone rubber is an elastomer (rubber-like material) composed of silicone —itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost. Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from − 55 to 300 °C (− 67 to 572 °F) while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including: voltage line insulators, automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware with products such as silicone sealants.

Curing In its uncured state, silicone rubber is a highlyadhesive gel or liquid. In order to convert to a solid, it must be cured, vulcanized, or catalyzed. This is normally carried out in a two-stage process at the point of manufacture into the desired shape, and then in a prolonged post-cure process. It can also be injection molded. Silicone rubber may be cured by a platinumcatalyzed cure system, a condensation cure system, a peroxide cure system, or an oxime cure system. For the platinumcatalyzed cure system, the curing process can be accelerated by adding heat or pressure.
Platinum-based cure system In a platinum-based silicone cure system, also called an addition system (because the key reaction-building polymer is an addition reaction), a hydrideand a vinylfunctional siloxane polymer react in the presence of a platinum complex catalyst, creating an ethyl bridge between the two. [1] The reaction has no byproducts. Such silicone rubbers cure quickly, though the rate of or even ability to cure is easily inhibited in the presence of elemental tin, sulfur, and many amine compounds.

Condensation cure system Condensation curing systems can be one-part or twopart systems. In one-part or RTV (room-temperature vulcanizing) system, a cross-linker exposed to ambient humidity (i. e. , water) experiences a hydrolysis step and is left with a hydroxyl or silanol group. The silanol condenses further with another hydrolyzable group on the polymer or cross-linker and continues until the system is fully cured. Such a system will cure on its own at room temperature and (unlike the platinum-based addition cure system) is not easily inhibited by contact with other chemicals, though the process may be affected by contact with some plastics or metals and may not take place at all if placed in contact with already-cured silicone compounds.
Condensation cure system The crosslinkers used in condensation cure systems are typically alkoxy, acetoxy, ester, enoxy or oxime silanes such as methyl trimethoxy silane for alkoxy-curing systems and methyl triacetoxysilane for acetoxy-curing systems. In many cases an additional condensation catalyst is added to fully cure the RTV system and achieve a tack-free surface. Organotitanate catalysts such as tetraalkoxy titanates or chelated titanates are used in alkoxy-cured systems. Tin catalysts such as dibutyl tin dilaurate (DBTDL) can be used in oxime and acetoxy-cured systems. Acetoxy tin condensation is one of the oldest cure chemistries used for curing silicone rubber, and is the one used in household bathroom caulk. Depending on the type of detached molecule, it is possible to classify silicone systems as acidic, neutral or alkaline. [
Condensation cure system Two-part condensation systems package the crosslinker and condensation catalyst together in one part while the polymer and any fillers or pigments are in the second part. Mixing of the two parts causes the curing to take place. Once fully cured, condensation systems are effective as sealants and caulks in plumbing and building construction and as molds for casting polyurethane, epoxy and polyester resins, waxes, gypsum, and lowmelting-temperature metals such as lead. They are typically very flexible and have a high tear strength. They do not require the use of a release agent since silicones have non-stick properties.
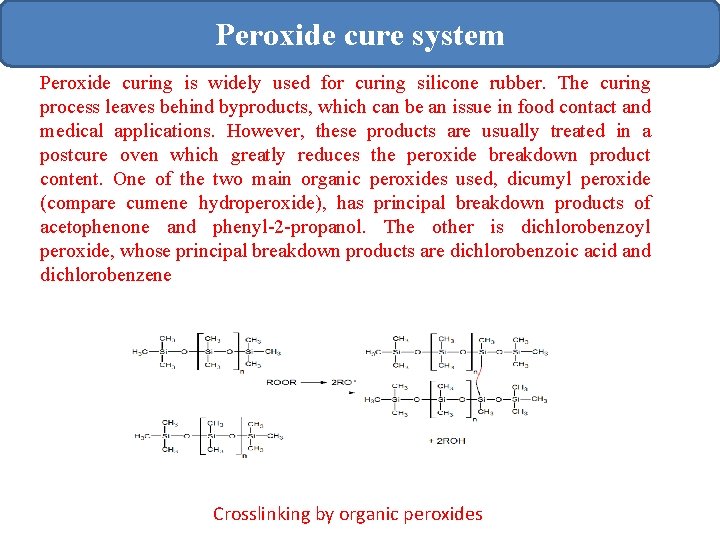
Peroxide cure system Peroxide curing is widely used for curing silicone rubber. The curing process leaves behind byproducts, which can be an issue in food contact and medical applications. However, these products are usually treated in a postcure oven which greatly reduces the peroxide breakdown product content. One of the two main organic peroxides used, dicumyl peroxide (compare cumene hydroperoxide), has principal breakdown products of acetophenone and phenyl-2 -propanol. The other is dichlorobenzoyl peroxide, whose principal breakdown products are dichlorobenzoic acid and dichlorobenzene Crosslinking by organic peroxides
- Slides: 12