MSc in High Performance Computing Computational Chemistry Module





- Slides: 36

MSc in High Performance Computing Computational Chemistry Module Introduction to Molecular Dynamics Bill Smith Computational Science and Engineering STFC Daresbury Laboratory Warrington WA 4 4 AD

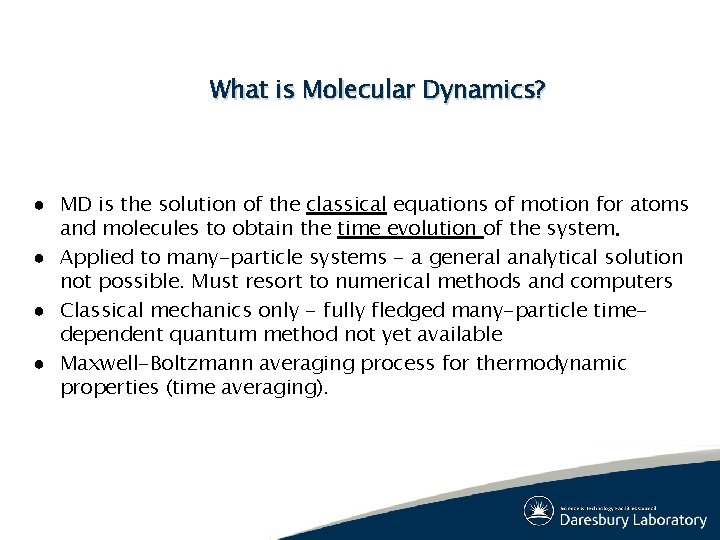
What is Molecular Dynamics? ● MD is the solution of the classical equations of motion for atoms and molecules to obtain the time evolution of the system. ● Applied to many-particle systems - a general analytical solution not possible. Must resort to numerical methods and computers ● Classical mechanics only - fully fledged many-particle timedependent quantum method not yet available ● Maxwell-Boltzmann averaging process for thermodynamic properties (time averaging).

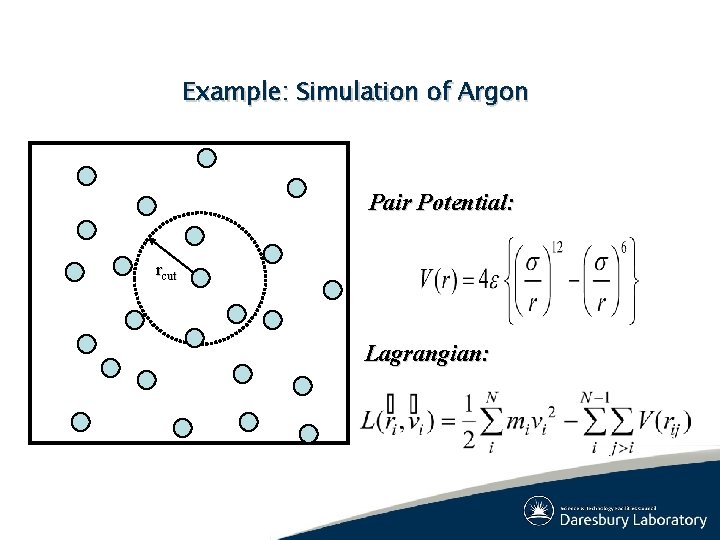
Example: Simulation of Argon Pair Potential: rcut Lagrangian:

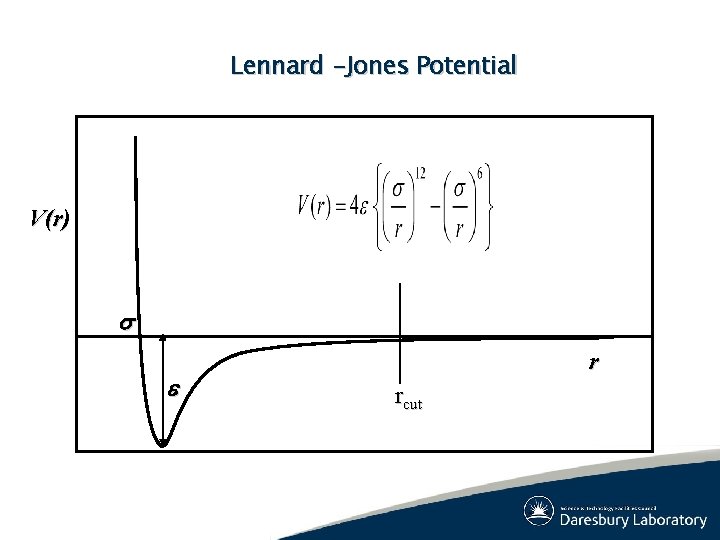
Lennard -Jones Potential V(r) s e r rcut

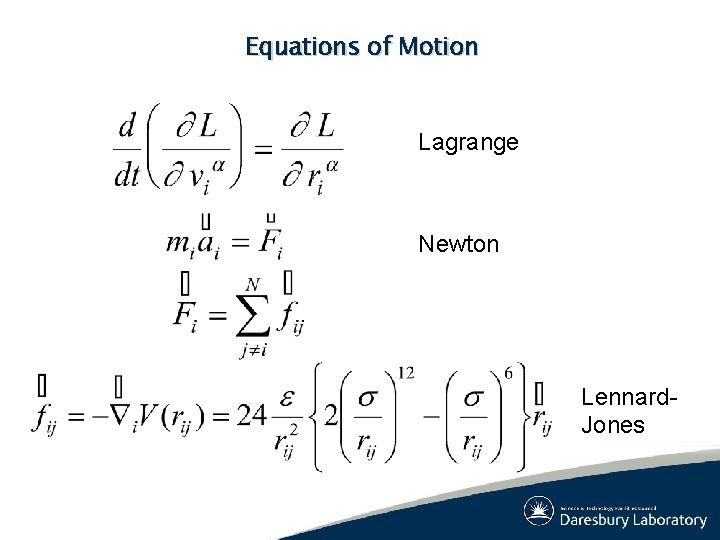
Equations of Motion Lagrange Newton Lennard. Jones

Periodic Boundary Conditions

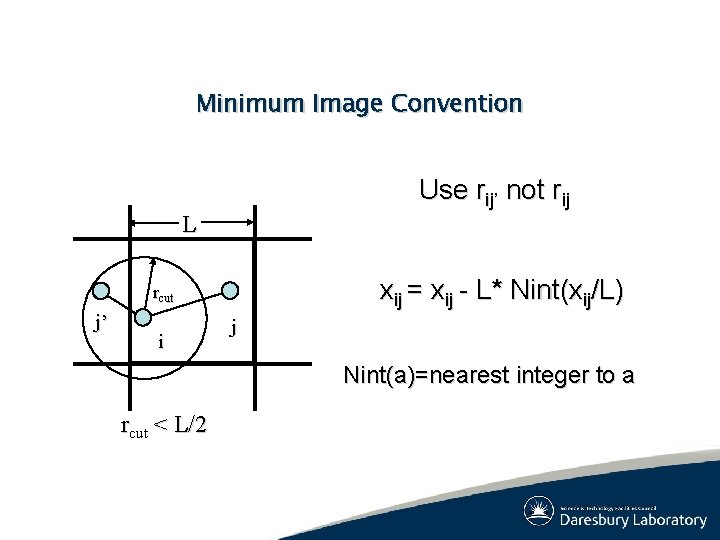
Minimum Image Convention Use rij’ not rij L xij = xij - L* Nint(xij/L) rcut j’ i j Nint(a)=nearest integer to a rcut < L/2

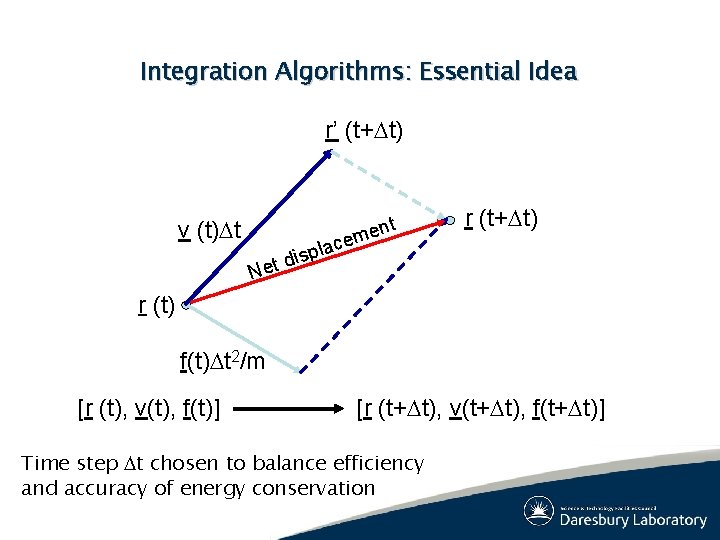
Integration Algorithms: Essential Idea r’ (t+Dt) v (t)Dt t n e em r (t+Dt) c la p s i d Net r (t) f(t)Dt 2/m [r (t), v(t), f(t)] [r (t+Dt), v(t+Dt), f(t+Dt)] Time step Dt chosen to balance efficiency and accuracy of energy conservation

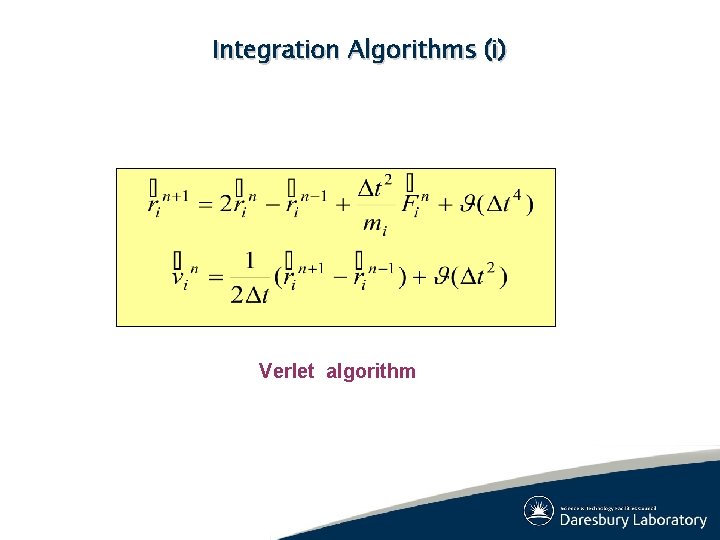
Integration Algorithms (i) Verlet algorithm

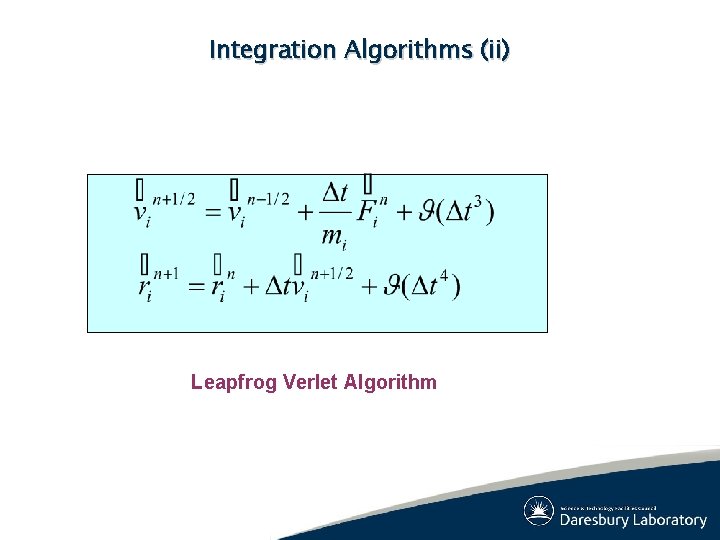
Integration Algorithms (ii) Leapfrog Verlet Algorithm

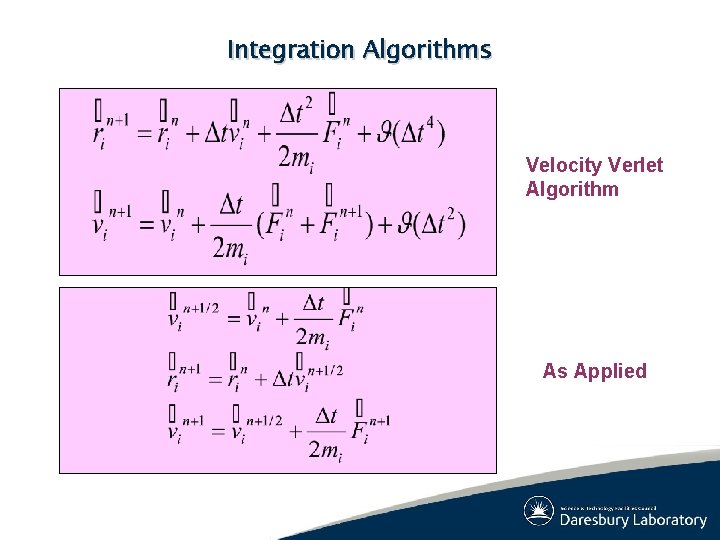
Integration Algorithms Velocity Verlet Algorithm As Applied

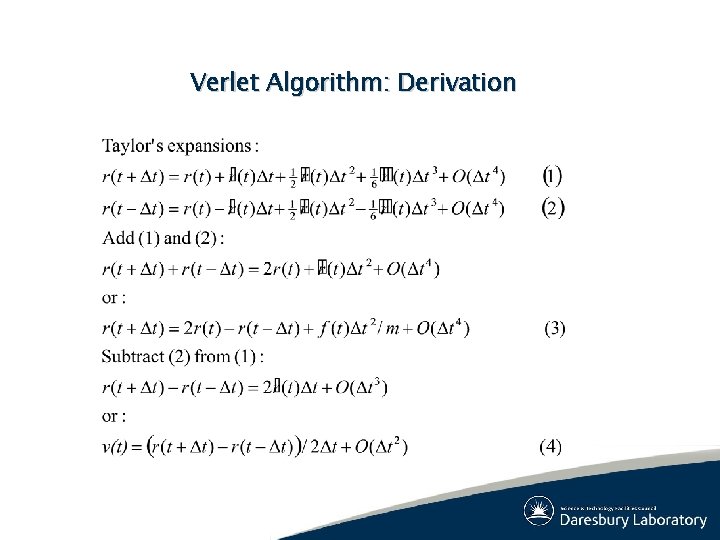
Verlet Algorithm: Derivation

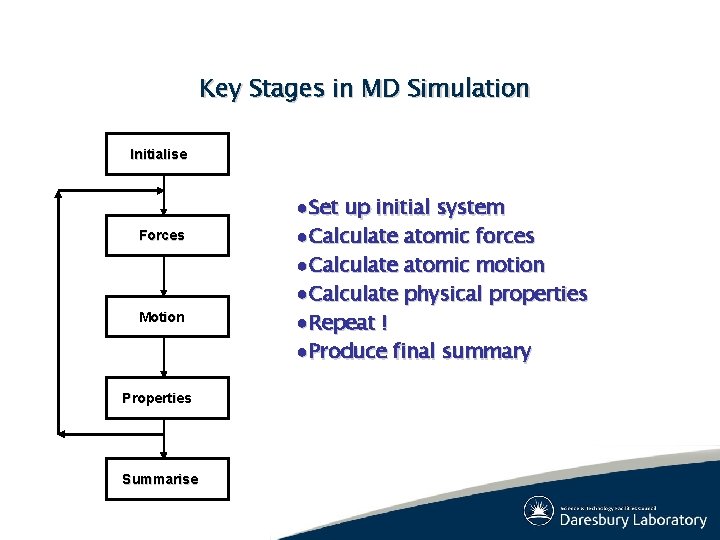
Key Stages in MD Simulation Initialise Forces Motion Properties Summarise ●Set up initial system ●Calculate atomic forces ●Calculate atomic motion ●Calculate physical properties ●Repeat ! ●Produce final summary

MD – Further Comments Constraints and Shake If certain motions are considered unimportant, constrained MD can be more efficient e. g. SHAKE algorithm - bond length constraints Rigid bodies can be used e. g. Eulers methods and quaternion algorithms Statistical Mechanics The prime purpose of MD is to sample the phase space of the statistical mechanics ensemble. Most physical properties are obtained as averages of some sort. Structural properties obtained from spatial correlation functions e. g. radial distribution function. Time dependent properties (transport coefficients) obtained via temporal correlation functions e. g. velocity autocorrelation function.

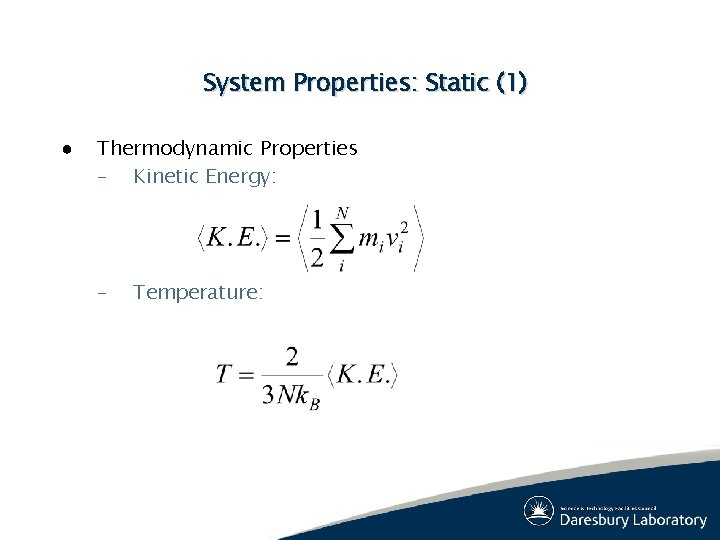
System Properties: Static (1) ● Thermodynamic Properties – Kinetic Energy: – Temperature:

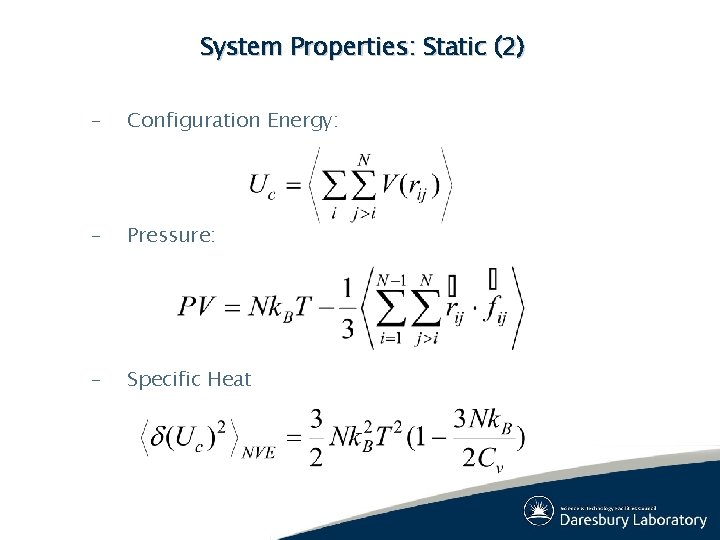
System Properties: Static (2) – Configuration Energy: – Pressure: – Specific Heat

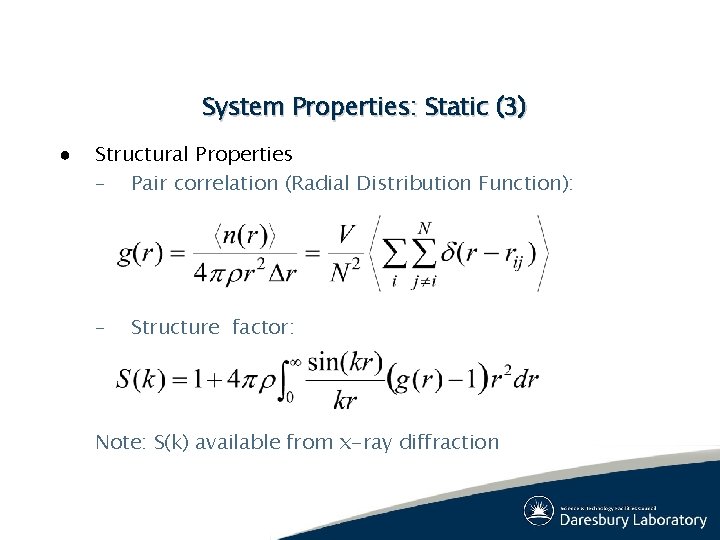
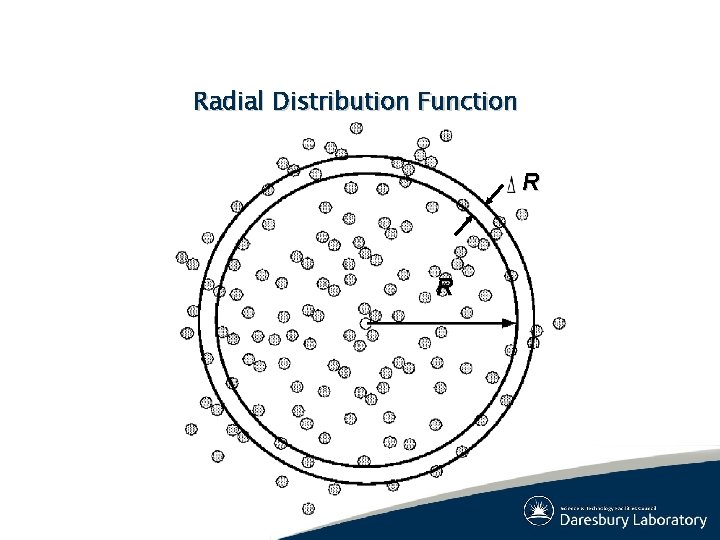
System Properties: Static (3) ● Structural Properties – Pair correlation (Radial Distribution Function): – Structure factor: Note: S(k) available from x-ray diffraction

Radial Distribution Function R R

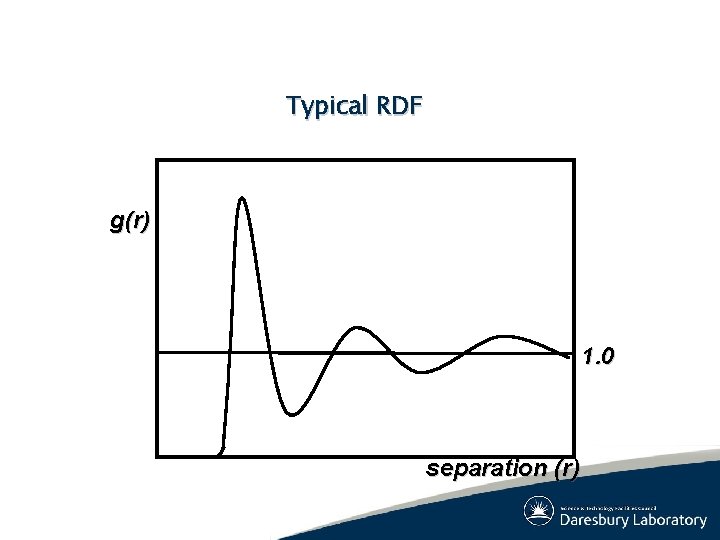
Typical RDF g(r) 1. 0 separation (r)

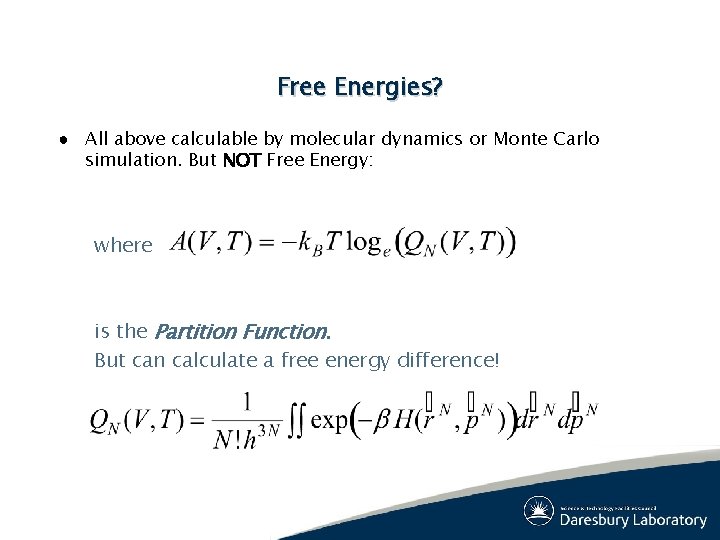
Free Energies? ● All above calculable by molecular dynamics or Monte Carlo simulation. But NOT Free Energy: where is the Partition Function. But can calculate a free energy difference!

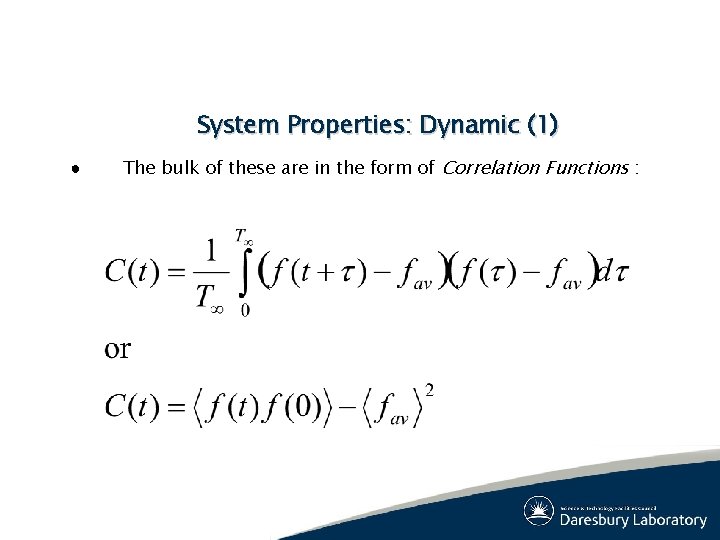
System Properties: Dynamic (1) ● The bulk of these are in the form of Correlation Functions :

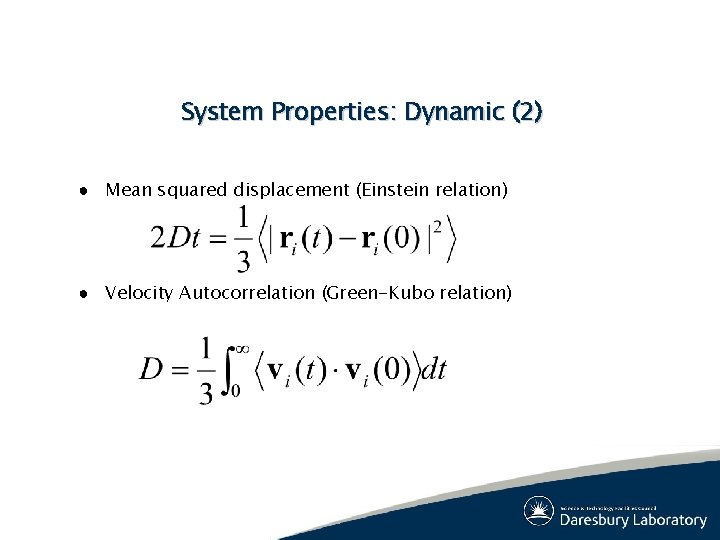
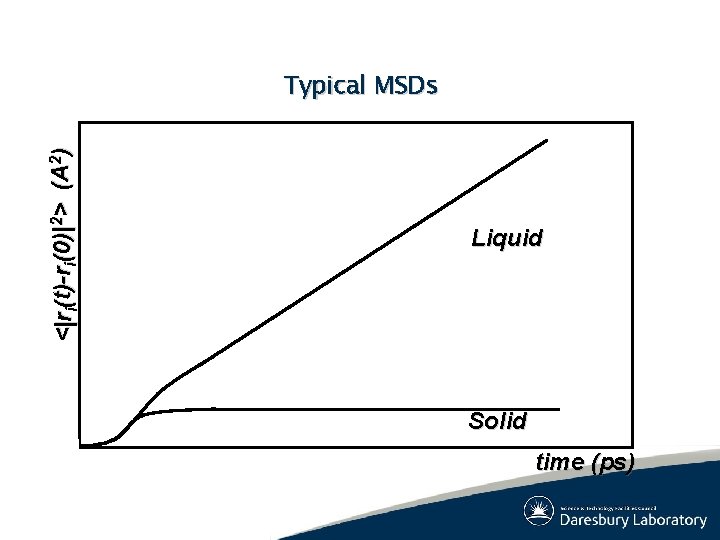
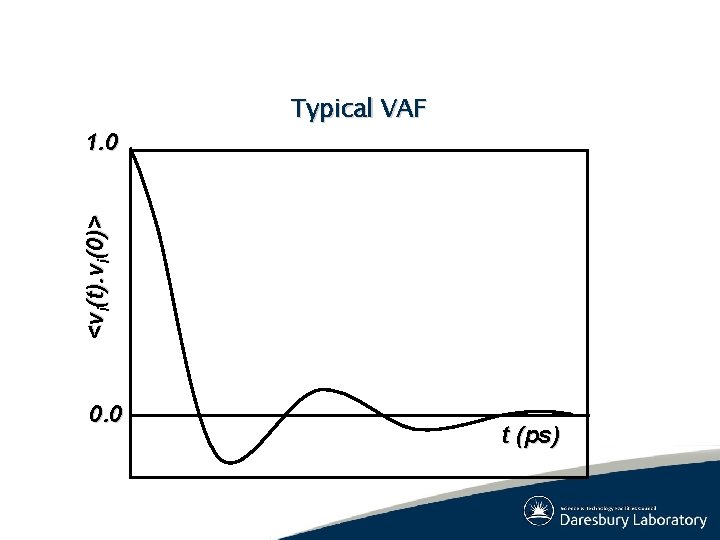
System Properties: Dynamic (2) ● Mean squared displacement (Einstein relation) ● Velocity Autocorrelation (Green-Kubo relation)

<|ri(t)-ri(0)|2> (A 2) Typical MSDs Liquid Solid time (ps)

<vi(t). vi(0)> 1. 0 Typical VAF 0. 0 t (ps)

Recommended Textbooks ● The Art of Molecular Dynamics Simulation, D. C. Rapaport, Camb. Univ. Press (2004) ● Understanding Molecular Simulation, D. Frenkel and B. Smit, Academic Press (2002). ● Computer Simulation of Liquids, M. P. Allen and D. J. Tildesley, Oxford (1989). ● Theory of Simple Liquids, J. -P. Hansen and I. R. Mc. Donald, Academic Press (1986). ● Classical Mechanics, H. Goldstein, Addison Wesley (1980)

The DL_POLY Package A General Purpose Molecular Dynamics Simulation Package

DL_POLY Background ● ● General purpose parallel MD code to meet needs of CCP 5 (academic collaboration) Authors W. Smith, T. R. Forester & I. Todorov Over 3000 licences taken out since 1995 Available free of charge (under licence) to University researchers.

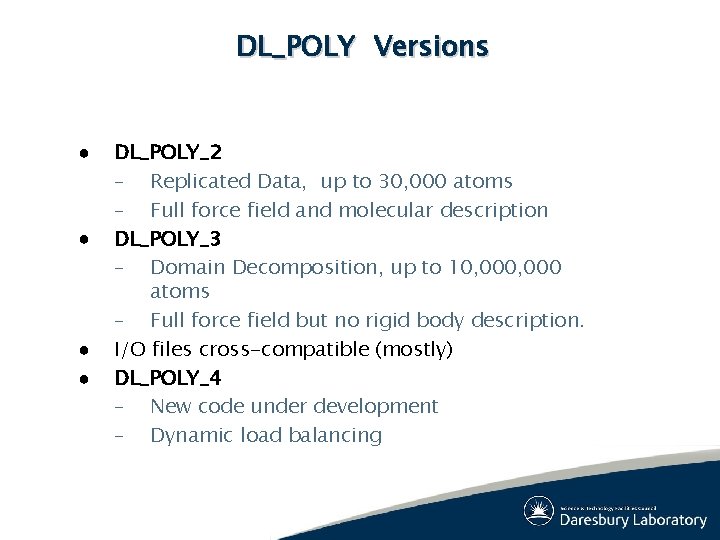
DL_POLY Versions ● ● DL_POLY_2 – Replicated Data, up to 30, 000 atoms – Full force field and molecular description DL_POLY_3 – Domain Decomposition, up to 10, 000 atoms – Full force field but no rigid body description. I/O files cross-compatible (mostly) DL_POLY_4 – New code under development – Dynamic load balancing

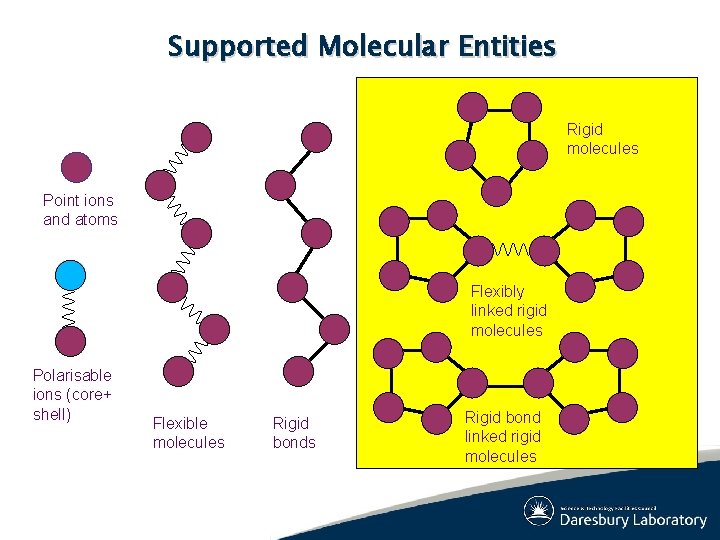
Supported Molecular Entities Rigid molecules Point ions and atoms Flexibly linked rigid molecules Polarisable ions (core+ shell) Flexible molecules Rigid bond linked rigid molecules

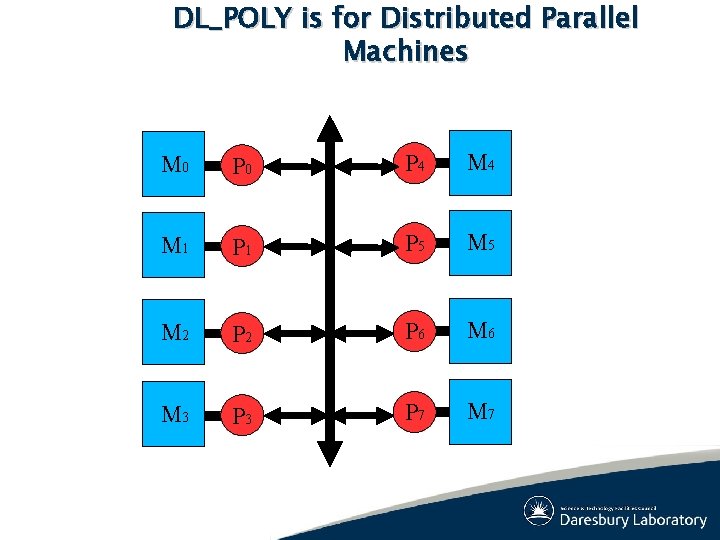
DL_POLY is for Distributed Parallel Machines M 0 P 4 M 1 P 5 M 2 P 6 M 3 P 7 M 7

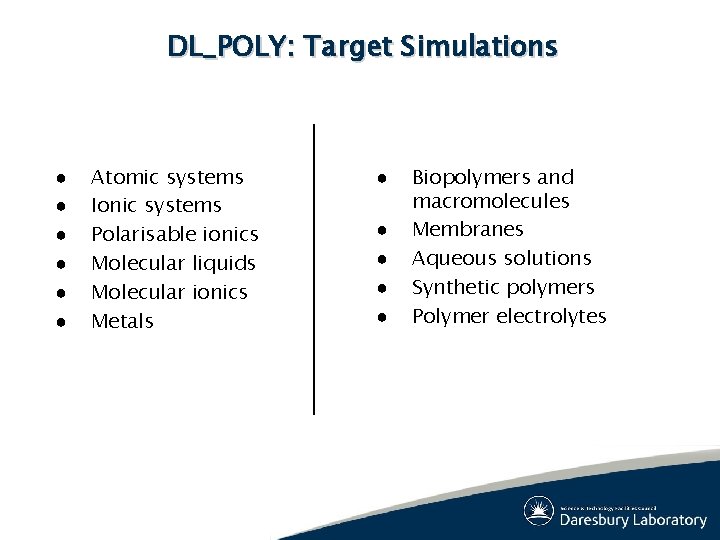
DL_POLY: Target Simulations ● ● ● Atomic systems Ionic systems Polarisable ionics Molecular liquids Molecular ionics Metals ● ● ● Biopolymers and macromolecules Membranes Aqueous solutions Synthetic polymers Polymer electrolytes

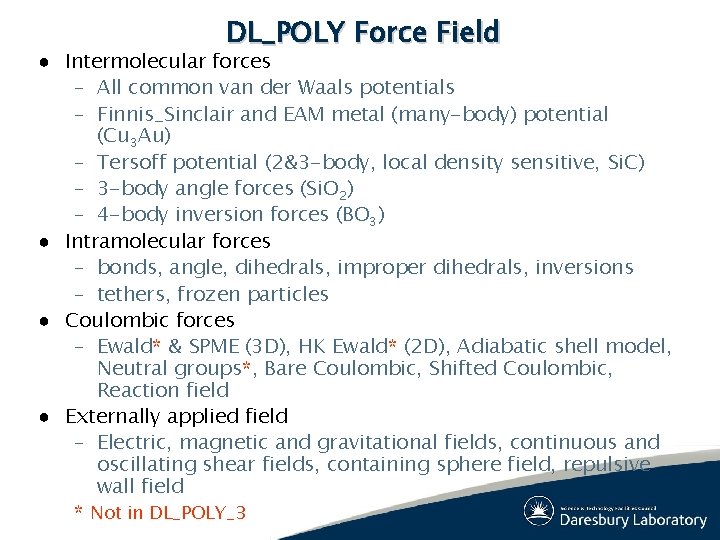
DL_POLY Force Field ● Intermolecular forces – All common van der Waals potentials – Finnis_Sinclair and EAM metal (many-body) potential (Cu 3 Au) – Tersoff potential (2&3 -body, local density sensitive, Si. C) – 3 -body angle forces (Si. O 2) – 4 -body inversion forces (BO 3) ● Intramolecular forces – bonds, angle, dihedrals, improper dihedrals, inversions – tethers, frozen particles ● Coulombic forces – Ewald* & SPME (3 D), HK Ewald* (2 D), Adiabatic shell model, Neutral groups*, Bare Coulombic, Shifted Coulombic, Reaction field ● Externally applied field – Electric, magnetic and gravitational fields, continuous and oscillating shear fields, containing sphere field, repulsive wall field * Not in DL_POLY_3

Algorithms and Ensembles Algorithms ● ● ● ● * Verlet leapfrog Velocity Verlet RD-SHAKE Euler-Quaternion* No_Squish* QSHAKE* [Plus combinations] Not in DL_POLY_3 Ensembles ● ● ● ● ● NVE Berendsen NVT Hoover NVT Evans NVT Berendsen NPT Hoover NPT Berendsen N T Hoover N T PMF

The DL_POLY Java GUI

The DL_POLY Website http: //www. ccp 5. ac. uk/DL_POLY/

The End