Ms Deanne Science 8 Solubility Viscosity and Density

Ms. Deanne Science 8 Solubility, Viscosity and Density

Solubility Lab � What did we learn from our lab? � What are some things we observed?

FACTORS AFFECTING SOLUBILITY � TYPE OF SOLVENT- Sugar will dissolve in water but not oil. � TYPE OF SOLUTE- More sugar will dissolve in 100 ml of water than salt � TEMPERATURE- for solids and liquids as you increase the temperature solubility increases. More sugar will dissolve in 100 C water than 30 C water.
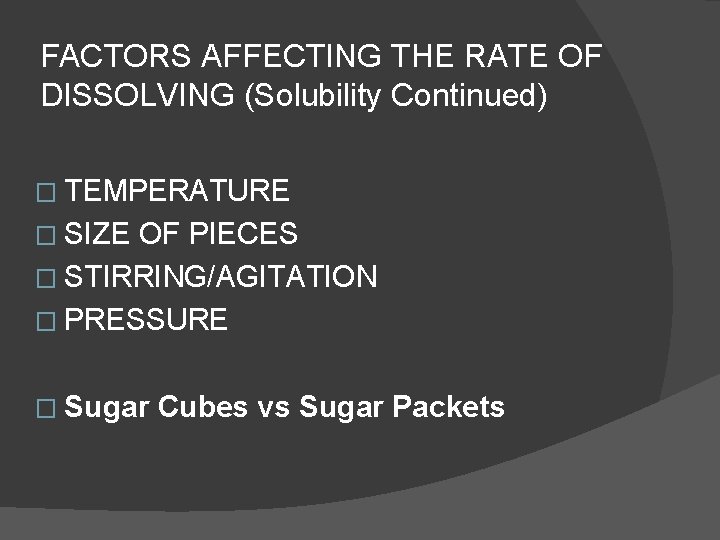
FACTORS AFFECTING THE RATE OF DISSOLVING (Solubility Continued) � TEMPERATURE � SIZE OF PIECES � STIRRING/AGITATION � PRESSURE � Sugar Cubes vs Sugar Packets

Super Saturated Solution � Rock Candy Demonstration �What is the Solute? �What is the Solvent? �Why can we add more solute? �What is affecting the Solubility and the Dissolving Rate?

From this point on, the material learned will be found on Quiz 2

VISCOSITY � Viscosity = thickness or resistance to flow � High viscosity – thick-honey, ketchup, syrup, molasses- high resistance � Low viscosity- thin-water, alcohol- low resistance

VISCOSITY MARBLE CHALLENEG There are 5 water bottles with 5 different liquids inside. � Controlled Variables: Water Bottle Type, marbles � Manipulated Variables: Liquids � �Dish Soap �Corn Syrup �Oil �Water �Mineral Oil

VISCOSITY MARBLE CHALLENGE � You are to time how long it takes for the marble to go from the bottom of the water bottle to the top. � You will be working in groups of 3 -4 for this. Record your times and answer the question below: �Why are the times different?
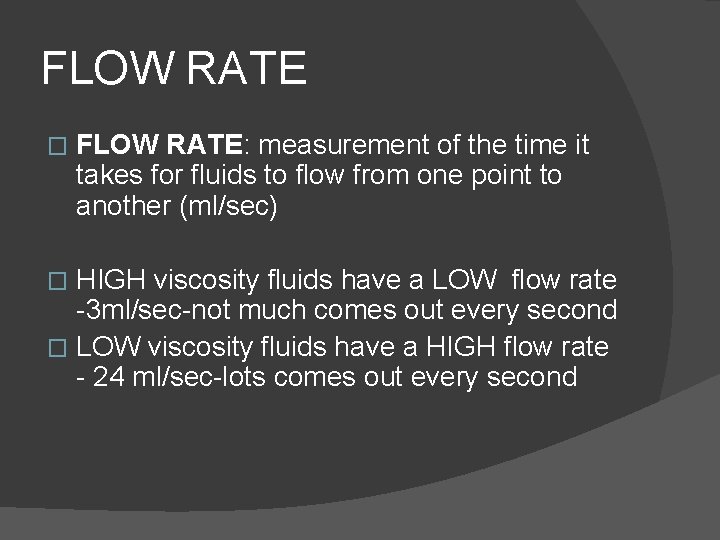
FLOW RATE � FLOW RATE: measurement of the time it takes for fluids to flow from one point to another (ml/sec) HIGH viscosity fluids have a LOW flow rate -3 ml/sec-not much comes out every second � LOW viscosity fluids have a HIGH flow rate - 24 ml/sec-lots comes out every second �

FLOW RATE � Methods to test viscosity and flow rate are: � The ramp method � The funnel method
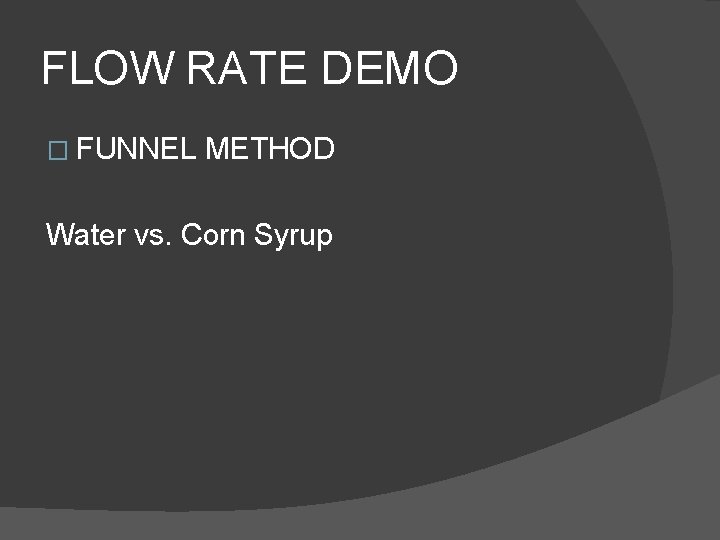
FLOW RATE DEMO � FUNNEL METHOD Water vs. Corn Syrup

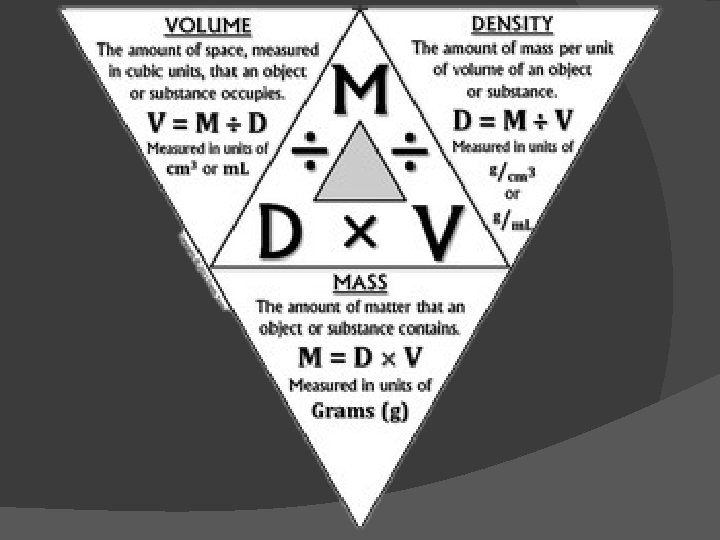
DENSITY AND FLUIDS � DENSITY: the mass per unit volume of a substance. Density is a more accurate measurement than just mass because it takes into account ‘how much’ of a substance there is. � D=m/v D= DENSITY (g/cm or kg/ m) � M= mass (g or kg) � V= volume (m. L, L or cm , m) �
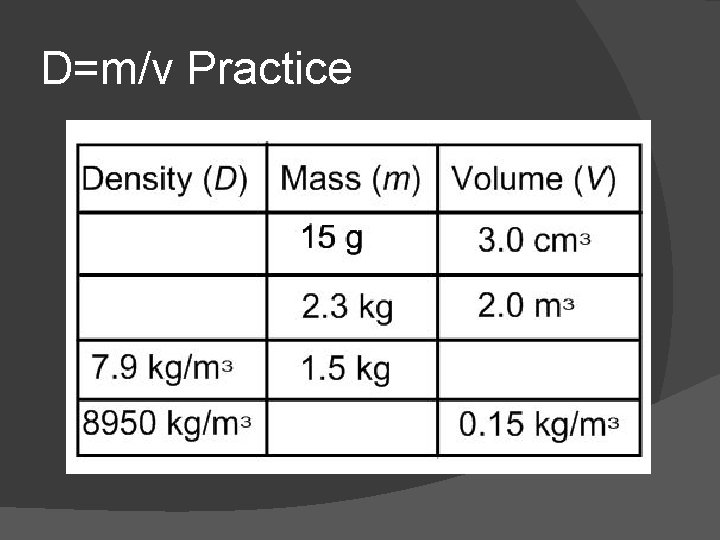
D=m/v Practice
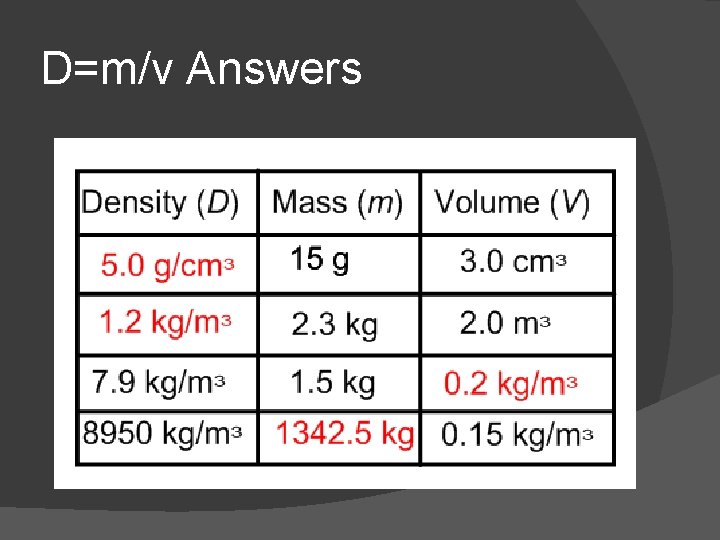
D=m/v Answers
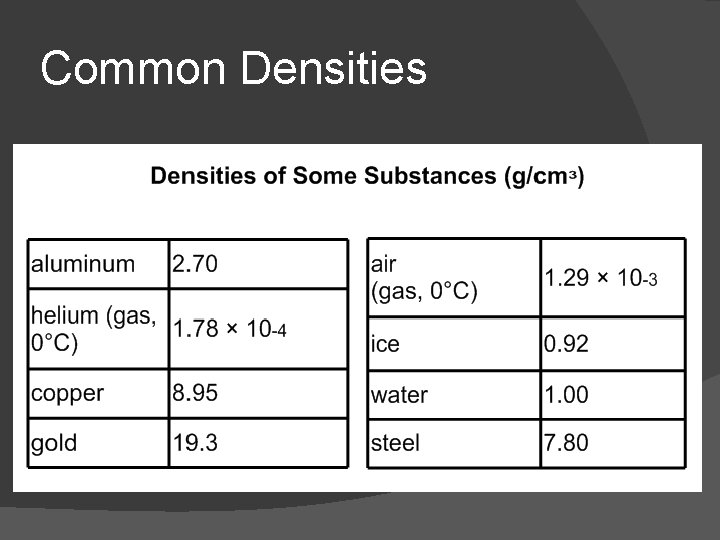
Common Densities
DENSITY SIMULATION � Density � What �A �B �C �D �E Simulation Ph. ET is the density of the objects?

Think About It! � You are walking along a rocky path, and you see a metallic, gold-colored rock. You estimate the mass to be 10 g and the volume to be 2 cm 3. � Is the rock gold?

Think About It! � Does � What a carrot sink or float in Tap Water? if we added salt to the water? Would the carrot still sink or float?
- Slides: 20