MS Calibration for Protein Profiles We need calibration
MS Calibration for Protein Profiles • We need calibration for – Accurate mass value • Mass error: • (Measured Mass – Theoretical Mass) X 106 ppm Theoretical Mass • 100 ppm is 1 Da mass error for a 10000 Da peak • For linear MALDI MS, the mass error is ~100 ppm – Proper binning
The MS generating process • Time-of-flight mass spectra are recorded as intensity versus time-of-flight tables. – (m/z) =(a. T+b)2 – a and b are defined by the physical instrument dimensions and the operating parameters • MS is assigned a default calibration that allows the interpretation of the spectrum. • Different conditions in the instrument – – Instrument errors in measuring high voltages and electrical field Time-varying high potentials Instrument parameters are subject to drift Different initial kinetic energy

"external" calibration • Applying a set of calibration constants measured in a previous experiment is known as using an "external" calibration. • External calibrations are not always accurate. – experimental conditions change and may produce a systematic error in mass assignment
"internal" calibration • This problem can be corrected using known peaks in each spectrum to calculate a calibration for that particular data set, a procedure known as using an "internal" calibration. – Internal calibrations are inherently more accurate than external calibrations; however they require at least two peaks of accurately known mass in every spectrum

"internal" calibration • It must be pointed out that good mass assignments depend on good signals. • The inconsistency of calibrant peak resolution and peak shape cause the inconsistency of internal calibration
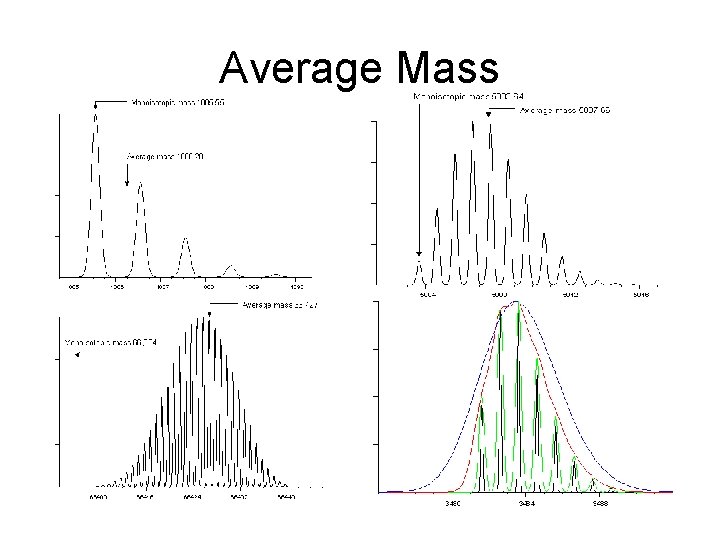
Average Mass
Choosing Calibrant Peaks • There are errors in estimating the precise centroid of the full isotopic envelope. • Isotope distributions are not symmetric, so it is essential to calculate a centroid, rather than just taking the apex of the distribution. • The accuracy of a centroid depends on the precise measurement of the ion current at a sufficient number of points to define the isotopic envelope precisely. • Factors which can distort relative intensities across the distribution and can contribute to error in the average mass value include – – overlapping distributions low ion statistics, detector saturation others
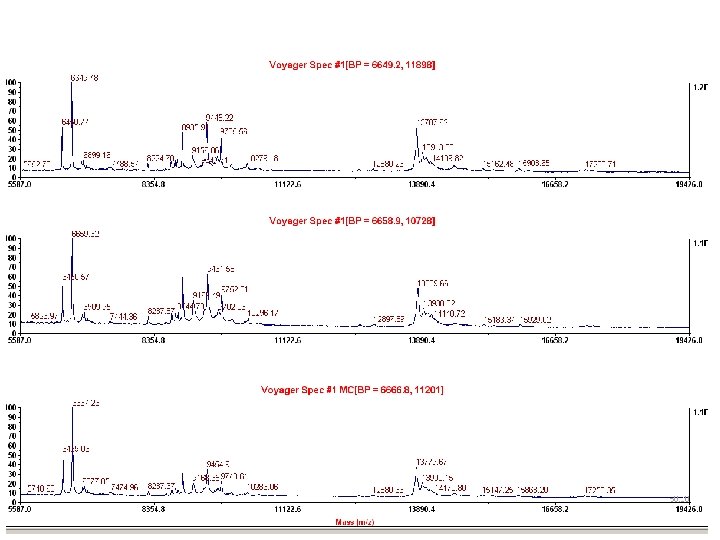
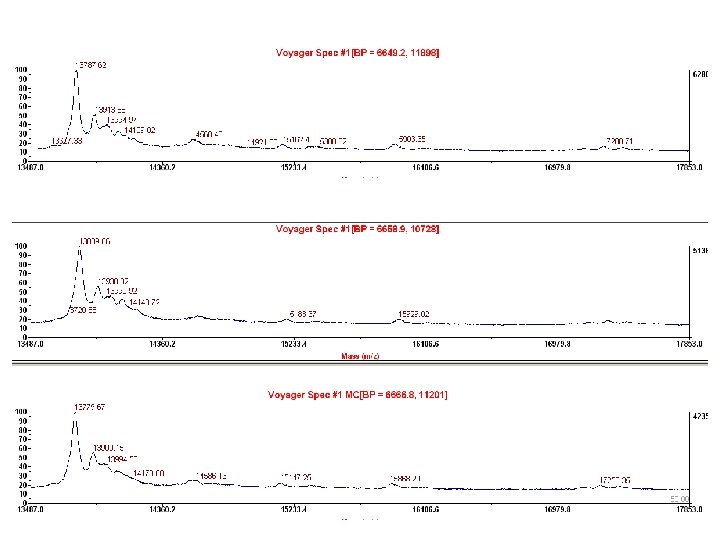
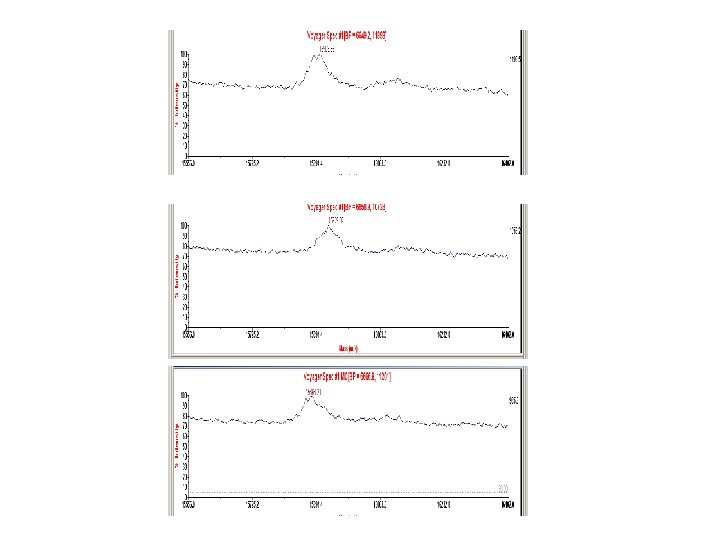

Choosing Calibrant Peaks • Consistently present in most spectra – Obtain multiple calibrant peaks • Good peak resolution – Accurate mass value assignment – Smooth MS before calibration • Known m/z value
- Slides: 11