MS 1 5 Representing Chemical Bonds Chemistry 30

MS 1. 5 Representing Chemical Bonds Chemistry 30 Mr. Goller
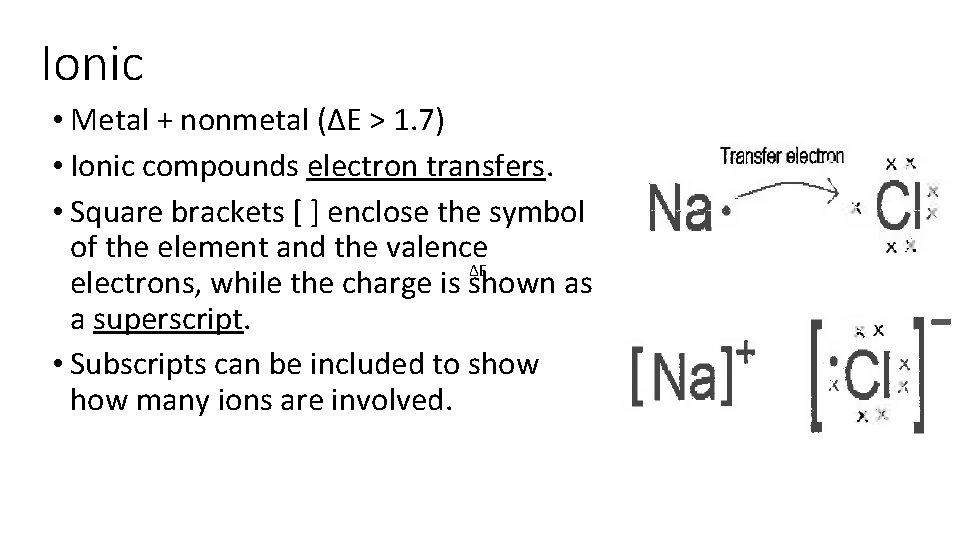
Ionic • Metal + nonmetal (ΔE > 1. 7) • Ionic compounds electron transfers. • Square brackets [ ] enclose the symbol of the element and the valence ΔE electrons, while the charge is shown as a superscript. • Subscripts can be included to show many ions are involved.
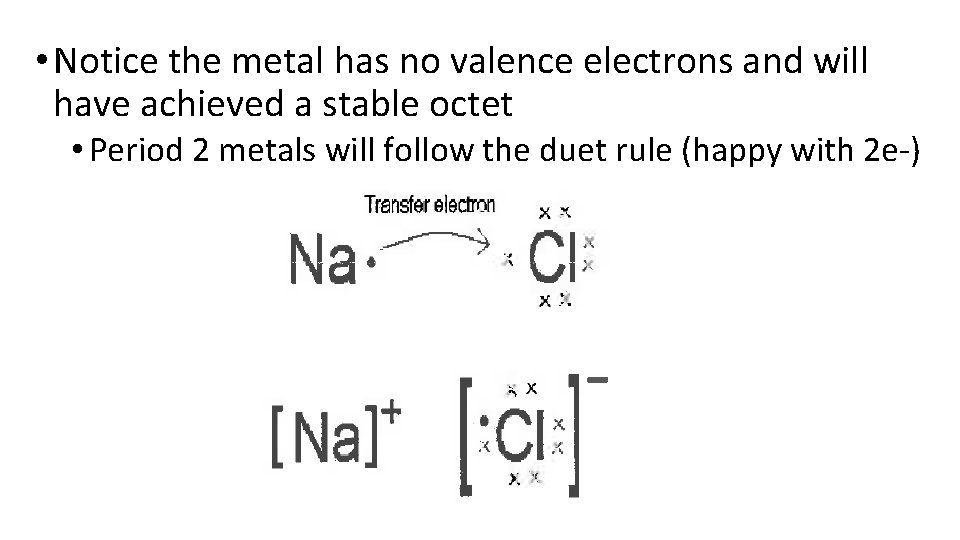
• Notice the metal has no valence electrons and will have achieved a stable octet • Period 2 metals will follow the duet rule (happy with 2 e-)
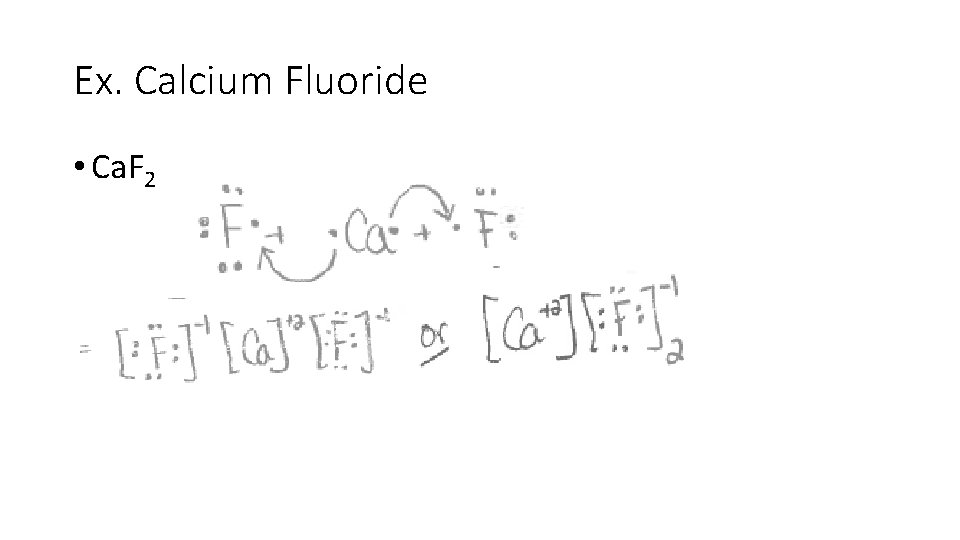
Ex. Calcium Fluoride • Ca. F 2
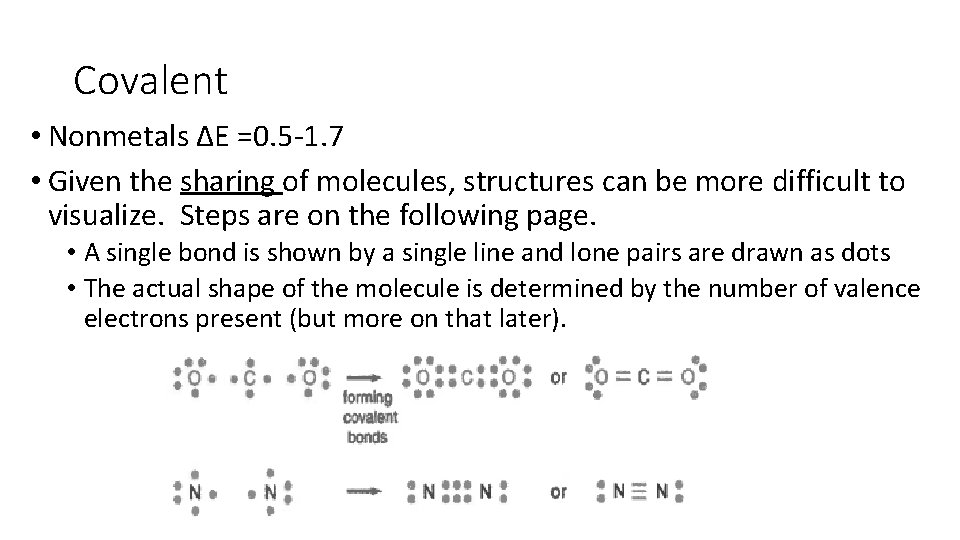
Covalent • Nonmetals ΔE =0. 5 -1. 7 • Given the sharing of molecules, structures can be more difficult to visualize. Steps are on the following page. • A single bond is shown by a single line and lone pairs are drawn as dots • The actual shape of the molecule is determined by the number of valence electrons present (but more on that later).
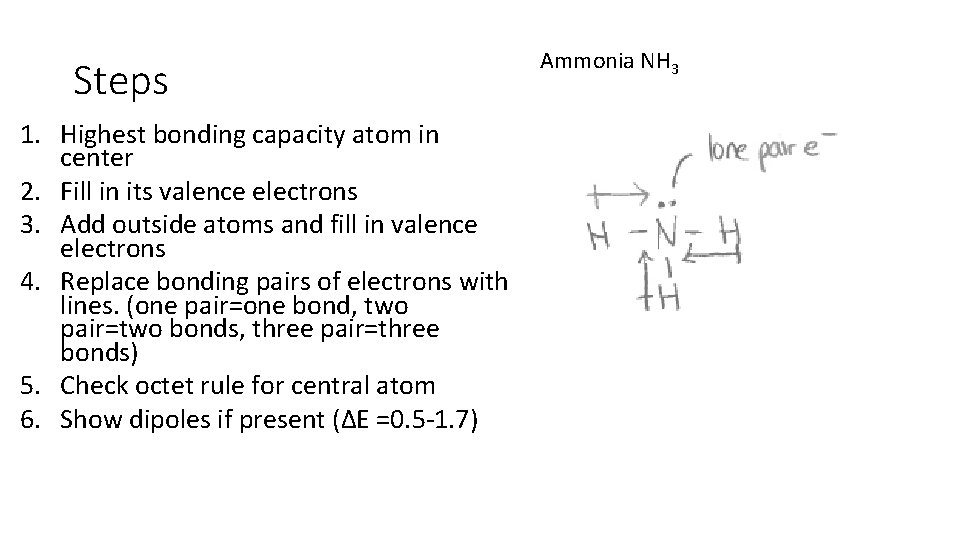
Steps 1. Highest bonding capacity atom in center 2. Fill in its valence electrons 3. Add outside atoms and fill in valence electrons 4. Replace bonding pairs of electrons with lines. (one pair=one bond, two pair=two bonds, three pair=three bonds) 5. Check octet rule for central atom 6. Show dipoles if present (ΔE =0. 5 -1. 7) Ammonia NH 3

Carbon Disulfide • CS 2
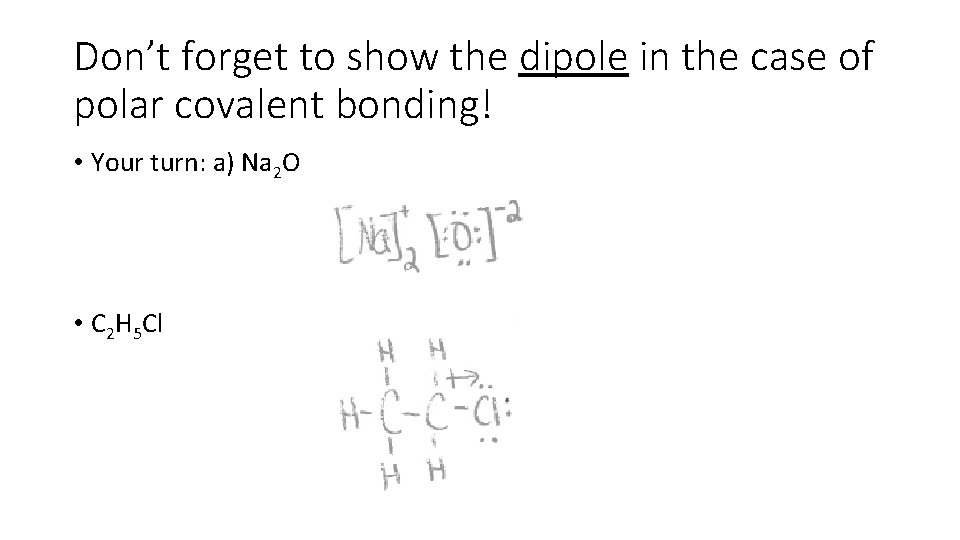
Don’t forget to show the dipole in the case of polar covalent bonding! • Your turn: a) Na 2 O • C 2 H 5 Cl
- Slides: 8