MRI Compatibility Device Patient Access to StateoftheArt Diagnostics

MRI Compatibility: Device Patient Access to State-of-the-Art Diagnostics Brian Urke Vice President & Brady General Manager Medtronic CRDM

4 Million Pacemaker Patients Worldwide 50 -75% will likely need an MRI* 70% of SCS patients will likely need an MRI over the average lifespan of an SCS device** *Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients, PACE. April 2005; 28(4): 326 -328 **Market. Scan® Commercial Claims and Encounters Database, 2008. Data Compiled from Truven Health Analytics, Inc.
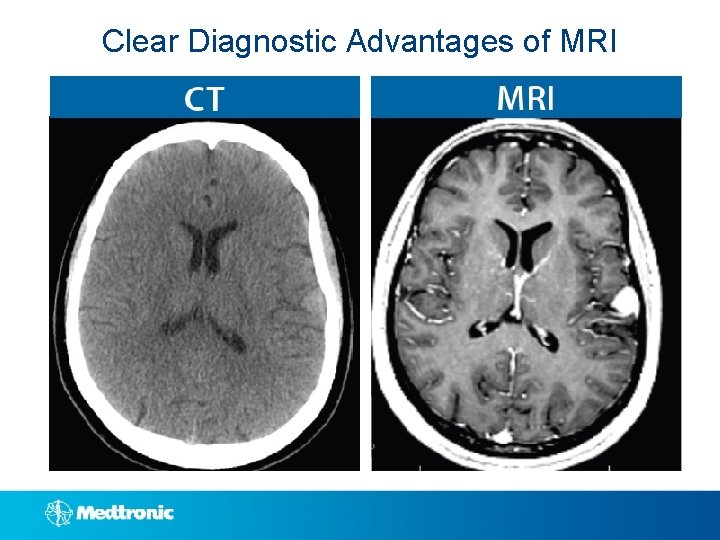
Clear Diagnostic Advantages of MRI
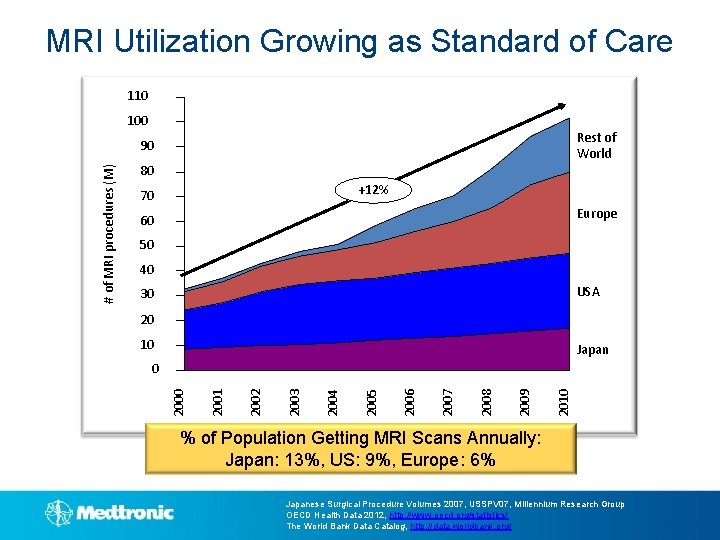
MRI Utilization Growing as Standard of Care 110 100 Rest of World 80 +12% 70 Europe 60 50 40 USA 30 20 10 Japan 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 0 2000 # of MRI procedures (M) 90 % of Population Getting MRI Scans Annually: Japan: 13%, US: 9%, Europe: 6% Japanese Surgical Procedure Volumes 2007, USSPV 07, Millennium Research Group OECD Health Data 2012, http: //www. oecd. org/statistics/ The World Bank Data Catalog, http: //data. worldbank. org/

Medtronic MRI Innovation Leadership Initiated Research 1997 1999 MR Conditional Labeling for Pumps 2001 2003 2005 MR Conditional Labeling for DBS MR Conditional Labeling for SCS MR Conditional Labeling for Reveal ILR 2007 2009 En. Rhythm MRI MR Conditional Pacemaker (EU) Revo MRI MR Conditional Pacemaker (US) 2011 2013 MR Conditional Labeling for Inter. Stim

MRI Environment: Powerful Fields

MRI: A Complex Environment for Medical Devices Static Field Coil 30, 000 x Earth’s magnetic field Gradient Coils Creates loud scanner noise Radio Frequency (RF) Coil 1000’s of watts peak power
Ensuring Device System Safety
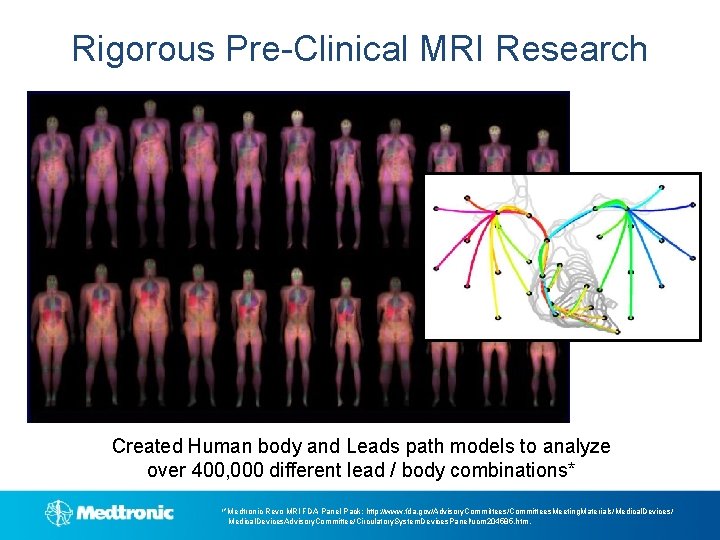
Rigorous Pre-Clinical MRI Research Created Human body and Leads path models to analyze over 400, 000 different lead / body combinations* 1*Medtronic Revo MRI FDA Panel Pack: http: //www. fda. gov/Advisory. Committees/Committees. Meeting. Materials/Medical. Devices/ Medical. Devices. Advisory. Committee/Circulatory. System. Devices. Panel/ucm 204585. htm.
Awareness of the Need for MRI & MR Conditional Devices • MRI provides critical insight that other diagnostics alternatives cannot match • Broad demand need for Device Patient access to MRI across healthcare specialties • MR Conditional Devices offer FDA approved MRI access
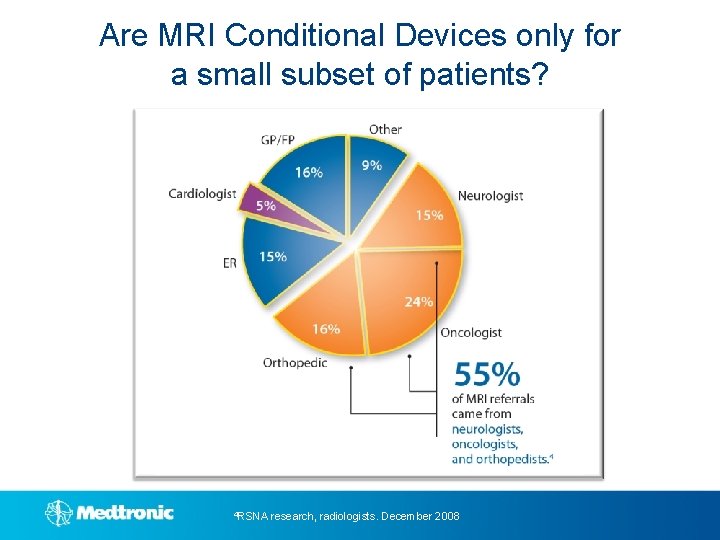
Are MRI Conditional Devices only for a small subset of patients? 4 RSNA research, radiologists. December 2008
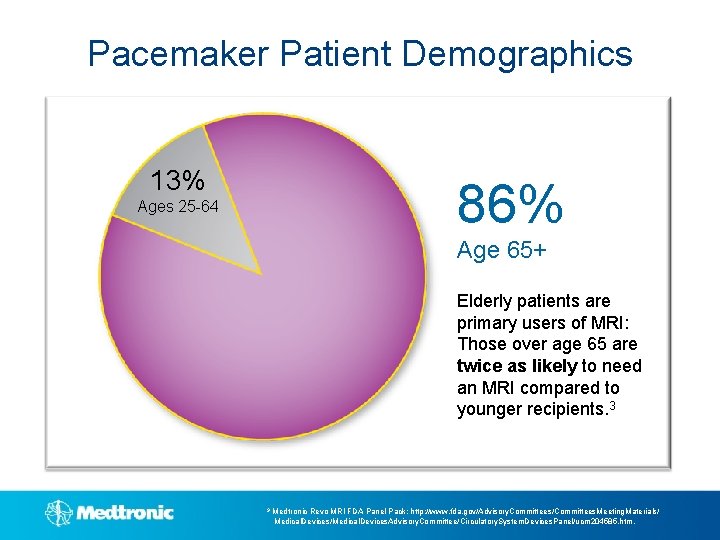
Pacemaker Patient Demographics 13% 86% Ages 25 -64 Age 65+ Elderly patients are primary users of MRI: Those over age 65 are twice as likely to need an MRI compared to younger recipients. 3 3 Medtronic Revo MRI FDA Panel Pack: http: //www. fda. gov/Advisory. Committees/Committees. Meeting. Materials/ Medical. Devices/Medical. Devices. Advisory. Committee/Circulatory. System. Devices. Panel/ucm 204585. htm.
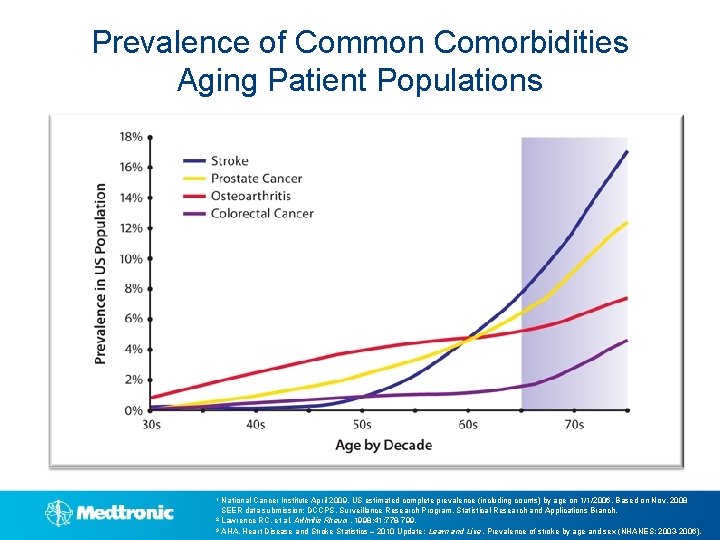
Prevalence of Common Comorbidities Aging Patient Populations National Cancer Institute April 2009. US estimated complete prevalence (including counts) by age on 1/1/2006. Based on Nov. 2008 SEER data submission; DCCPS, Surveillance Research Program, Statistical Research and Applications Branch. 2 Lawrence RC, et al. Arthritis Rheum. 1998; 41: 778 -799. 3 AHA. Heart Disease and Stroke Statistics – 2010 Update: Learn and Live. Prevalence of stroke by age and sex (NHANES: 2003 -2006). 1
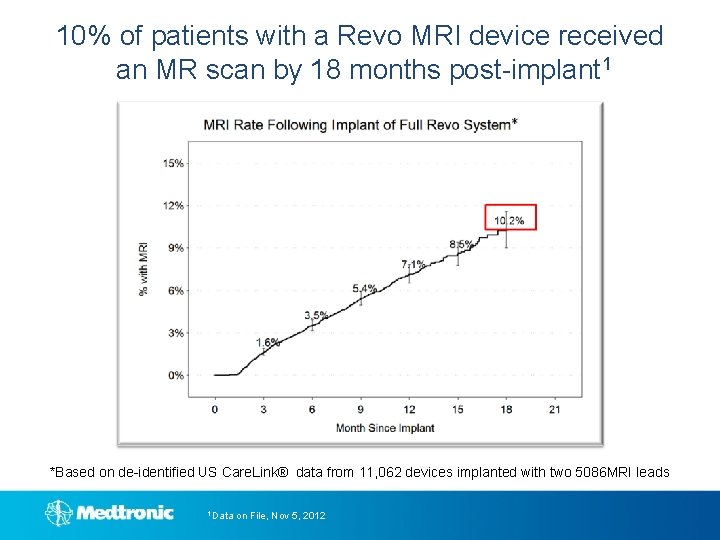
10% of patients with a Revo MRI device received an MR scan by 18 months post-implant 1 *Based on de-identified US Care. Link® data from 11, 062 devices implanted with two 5086 MRI leads 1 Data on File, Nov 5, 2012
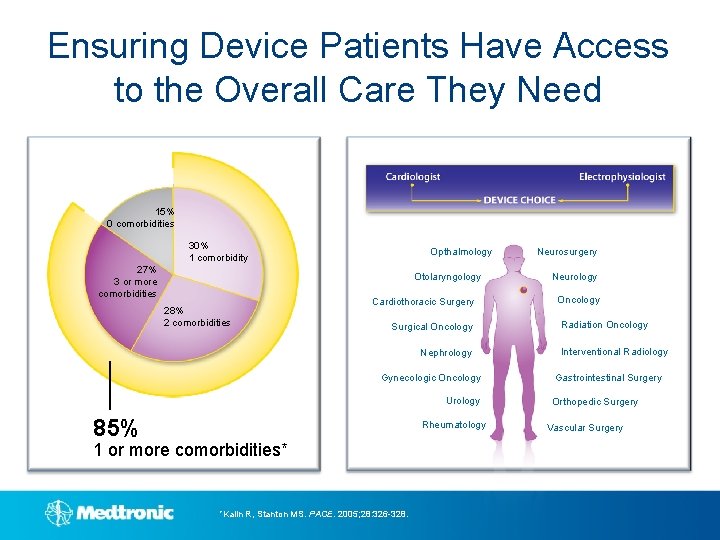
Ensuring Device Patients Have Access to the Overall Care They Need 15% 0 comorbidities 30% 1 comorbidity 27% 3 or more comorbidities Opthalmology Otolaryngology Cardiothoracic Surgery 28% 2 comorbidities Surgical Oncology Nephrology Gynecologic Oncology Urology 85% Rheumatology 1 or more comorbidities* *Kalin R, Stanton MS. PACE. 2005; 28: 326 -328. Neurosurgery Neurology Oncology Radiation Oncology Interventional Radiology Gastrointestinal Surgery Orthopedic Surgery Vascular Surgery

“Understanding the Potential Effects of MRI on Patients with Spinal Cord Stimulation Systems” Poster #244 Presented by Dr. Yair Safriel Neuroradiologist at Pharmascan, and University of South Florida, Clearwater, FL
- Slides: 16