Mr Kinton Honors Chemistry Review of Gases What





















- Slides: 29

Mr. Kinton Honors Chemistry

Review of Gases • What can you tell me about gases? • How are they similar to solids and liquids? • How are they different?

Characteristics of Gases • Expand spontaneously to fill their container • Volume of a gas is equal to the volume of the container • Highly compressible • Form homogeneous mixtures only • Molecules are spaced far from each other

Properties of importance for Gases • Measurable quantities include • Temperature • Volume • Pressure • For now we are going to focus on pressure • Force that acts on a certain area or P= F/A

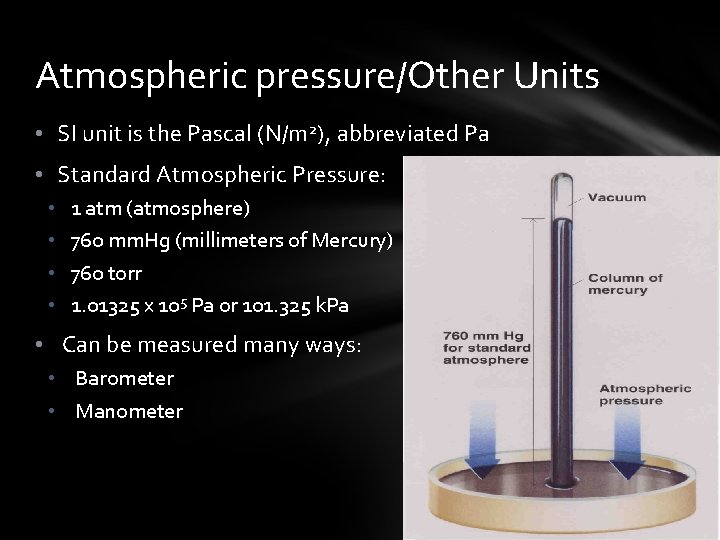
Atmospheric pressure/Other Units • SI unit is the Pascal (N/m 2), abbreviated Pa • Standard Atmospheric Pressure: • • 1 atm (atmosphere) 760 mm. Hg (millimeters of Mercury) 760 torr 1. 01325 x 105 Pa or 101. 325 k. Pa • Can be measured many ways: • Barometer • Manometer

Conversions • Convert. 357 atm to torr • Convert 6. 6 x 10 -2 torr to atm • Convert 147. 2 k. Pa to mm. Hg

Gas Laws • In order to understand gases we need can examine 4 key quantities: • • Temperature (T) Pressure (P) Volume (V) Amount of gas (n) • Scientists used these 4 to develop the gas laws

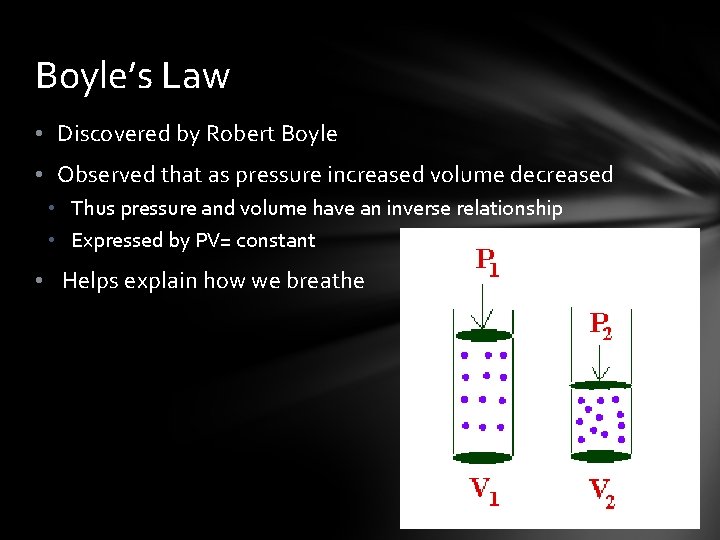
Boyle’s Law • Discovered by Robert Boyle • Observed that as pressure increased volume decreased • Thus pressure and volume have an inverse relationship • Expressed by PV= constant • Helps explain how we breathe

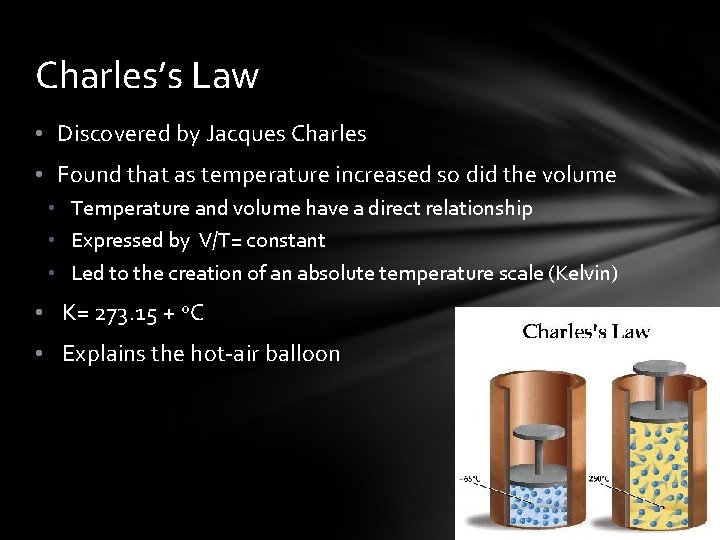
Charles’s Law • Discovered by Jacques Charles • Found that as temperature increased so did the volume • Temperature and volume have a direct relationship • Expressed by V/T= constant • Led to the creation of an absolute temperature scale (Kelvin) • K= 273. 15 + o. C • Explains the hot-air balloon

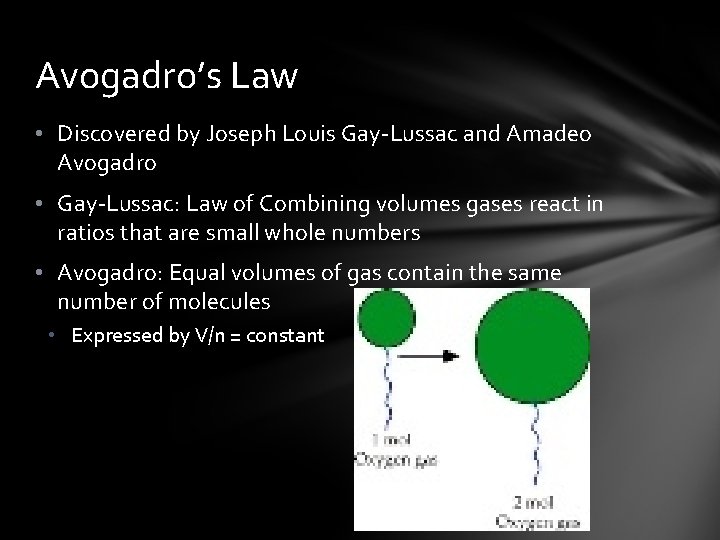
Avogadro’s Law • Discovered by Joseph Louis Gay-Lussac and Amadeo Avogadro • Gay-Lussac: Law of Combining volumes gases react in ratios that are small whole numbers • Avogadro: Equal volumes of gas contain the same number of molecules • Expressed by V/n = constant

Ideal Gas Law • Hypothetical gas whose behavior is described by the following equation: • PV= n. RT, where R is the gas constant • R=. 08206 L-atm/mol-K, 8. 314 m 3 -Pa/mol-K, 62. 36 L-torr/mol -K • Temperature must always be given in Kelvin • Gives us standard conditions: • Temperature: 0 o. C • Pressure: 1 atm

Example Calcium carbonate decomposes when heated and produces carbon dioxide. 250 m. L of carbon dioxide is collected. The pressure exerted is 1. 3 atm at a temperature of 31 o. C. How many moles of carbon dioxide were produced

Relating the Gas Laws • Due to the ideal gas law we can re-write the previous laws to determine how individual changes occur: • Boyle’s Law: P 1 V 1= P 2 V 2 • Charles’s Law: V 1/T 1= V 2/T 2 • Avogadro’s Law: V 1/n 1= V 2/n 2

Examples: A fixed quantity of gas at 23 degrees Celsius exhibits a pressure of 748 torr and occupies a volume of 10. 3 L. Calculate the volume if the pressure is increased to 1. 88 atm? Calculate the volume the gas will occupy if the temperature is increased to 165 degrees Celsius

Application of the Ideal Gas Law • We can use the ideal gas law to help us solve for 2 other physical properties of a gas: • Gas density • Molar mass • We can derive both of these quantities from the Ideal gas equation • d= PM/RT • M= d. RT/P

Example What is the density of carbon tetrachloride vapor at 714 torr and 125 o C?

Application of the Ideal Gas Law • We have seen in chemical reactions that gases can be reactants and products. • Using the ideal gas law we can determine the amount of gas that was consumed or produced in a chemical reaction. • Let’s examine how this works with the production of nitric acid. How many liters of ammonia at 850 o C and 5. 00 atm are required to react with 1. 00 moles of O 2(g)? • 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g)

You Try! The safety air bags in automobiles inflated by nitrogen gas generated by the rapid decomposition of sodium azide, Na. N 3: 2 Na. N 3(s) 2 Na(s) + 3 N 2(g). If an air bag has a volume of 36 L and is to be filled with nitrogen gas at a pressure of 1. 15 atm at a temperature of 26. 0 o C, how many grams of Na. N 3 must be decomposed?

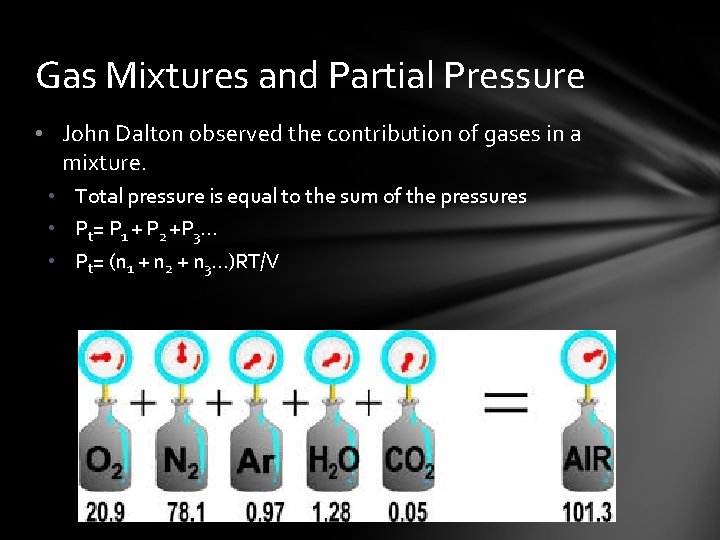
Gas Mixtures and Partial Pressure • John Dalton observed the contribution of gases in a mixture. • Total pressure is equal to the sum of the pressures • Pt= P 1 + P 2 +P 3… • Pt= (n 1 + n 2 + n 3…)RT/V

Example • A gaseous mixture made from 6. 00 g of O 2 and 9. 00 g CH 4 is placed in a 15. 0 -L vessel at 0 o. C. What is the partial pressure of each gas and what is the total pressure in the vessel.

Collecting a Gas over Water • Pt= Pgas + Pwater • Must use appendix B in the textbook to solve these problems

Example A sample of KCl. O 3 is decomposed and the gas is collected over water. The volume of gas collected is. 250 L at 26 o. C and 765 torr of total pressure. How many moles of O 2 are collected?

Kinetic-Molecular Theory • Ideal gas equation describes how gases behave but doesn’t explain why. • Rudolf Clausius described a satisfactory theory • A theory that is based on moving molecules

Theory of Moving Molecules 1. Gases consist of a large number of molecules in continuous, random motion 2. Volume of all molecules of the gas is negligible compared to the total volume 3. Attractive and repulsive forces are negligible 4. Energy can be transferred during collisions but the average kinetic energy does not change 5. Average kinetic energy is proportional to the absolute temperature. At constant temperature gas molecules have the same average kinetic energy

Real Gases: Deviate from Ideal Gases • In actuality gases do not behave like ideal gases • Deviate at high pressure • Deviate at lower temperatures • In actuality they occupy space and they do attract one another

Root-mean-square Speed • This is the speed of a gas molecule possessing average kinetic energy and denoted by the symbol u • u= √ 3 RT/M • Based on kinetic molecular theory we know that the average kinetic energy of any collection of gas molecules is ½ mu 2 • This shows us that lighter gases have a higher rms than heavier gases • Effusion- the escape of gas through a tiny hole into an evacuated space • Diffusion- the spread of one substance throughout a space or second substance

Example Calculate the rms, u, of N 2 at 25 o. C

Graham’s Law of Effusion • Shows that the effusion rate of a gas is inversely proportional to the square root of it’s molar mass • r 1/r 2= √M 2/M 1 • An unknown gas composed of diatomic molecules effuses at a rate that is only. 355 times that of O 2 at the same temperature. What is the identity of this unknown gas?

You Try! Calculate the ratio of the effusion rates of N 2 and O 2