Movement of Molecules Osmosis Diffusion Terms to Know










- Slides: 12

Movement of Molecules Osmosis & Diffusion

Terms to Know • Mixture - a substance consisting of two or more substances mixed together (not in fixed proportions and not with chemical bonding) • Homogeneous - describes a sample of matter that has uniform characteristics throughout • Heterogeneous - describes a sample of matter that has parts with different compositions

Terms to Know • Solution - a homogeneous mixture of two or more substances; frequently (but not necessarily) a liquid solution • Solute -a substance that is dissolved in another substance, thus forming a solution • Solvent -a substance that dissolves other substances, thus forming a solution

Terms to Know • Concentration - measure of how much of a given substance there is mixed with another substance; the number of molecules of a substance in a given volume • Concentration Gradient - change in the concentration of a substance from one area to another.

Terms to Know • Hypotonic – The solution on one side of a membrane where the solute concentration is less than on the other side. Hypotonic Solutions contain a low concentration of solute relative to another solution. • Hypertonic – The solution on one side of a membrane where the solute concentration is greater than on the other side. Hypertonic Solutions contain a high concentration of solute relative to another solution.

Terms to Know • Isotonic - Isotonic Solutions contain the same concentration of solute as an another solution

Concentrations http: //www. tvdsb. on. ca/westmin/sc ience/sbi 3 a 1/Cells/Osmosis. htm

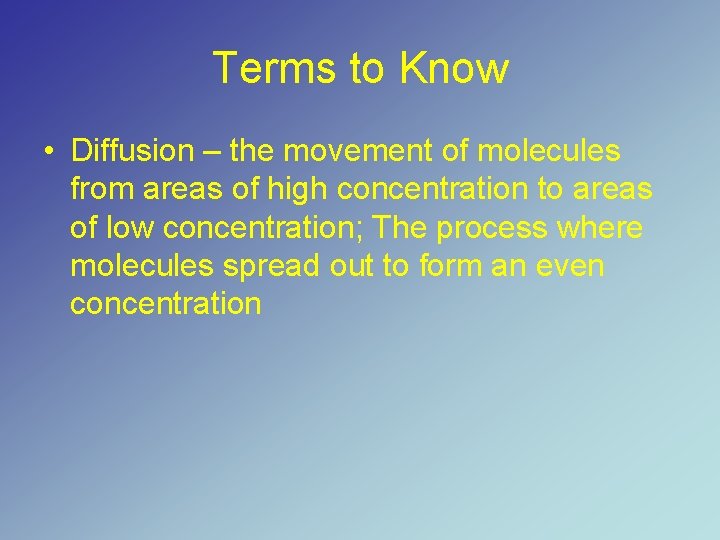
Terms to Know • Diffusion – the movement of molecules from areas of high concentration to areas of low concentration; The process where molecules spread out to form an even concentration

Diffusion Example http: //www. wisconline. com/objects/index_tj. asp? o bjid=AP 1903

Terms to Know • Osmosis – the diffusion of water across a selectively permeable membrane; the movement of a substance dissolved in a solution with a lower concentration through a membrane to a solution with a higher concentration • Selectively permeable - a barrier that allows some chemicals to pass but not others. The cell membrane is such a barrier.

Osmosis Example http: //www. wisconline. com/objects/index_tj. asp? o bjid=AP 11003

Sources • http: //www. tvdsb. on. ca/westmin/science/sbi 3 a 1/Cells/Os mosis. htm • www. shodor. org/master/environmental/water/runoff/Run off. Glossary. html • wordnet. princeton. edu/perl/webwn • wblrd. sk. ca/~chem 30_dev/appendix/glossary. htm • http: //www. wisconline. com/objects/index_tj. asp? objid=AP 1903 • www. emc. maricopa. edu/faculty/farabee/BIOBK/Bio. Book gloss. S. html • www. geocities. com/templarser/complexglos. html