Morphology of slags components by scanning microscopy Glaze

Morphology of slags components by scanning microscopy
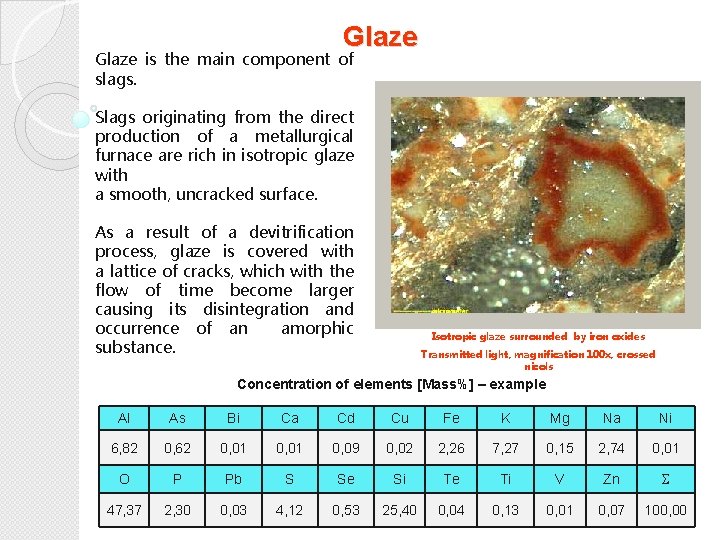
Glaze is the main component of slags. Slags originating from the direct production of a metallurgical furnace are rich in isotropic glaze with a smooth, uncracked surface. As a result of a devitrification process, glaze is covered with a lattice of cracks, which with the flow of time become larger causing its disintegration and occurrence of an amorphic substance. Isotropic glaze surrounded by iron oxides Transmitted light, magnification 100 x, crossed nicols Concentration of elements [Mass%] – example Al As Bi Ca Cd Cu Fe K Mg Na Ni 6, 82 0, 62 0, 01 0, 09 0, 02 2, 26 7, 27 0, 15 2, 74 0, 01 O P Pb S Se Si Te Ti V Zn 47, 37 2, 30 0, 03 4, 12 0, 53 25, 40 0, 04 0, 13 0, 01 0, 07 100, 00

Glaze Fot. XN, 100 x Glaze with hematite and magnetite Transmitted light, magnification 100 x, crossed nicols
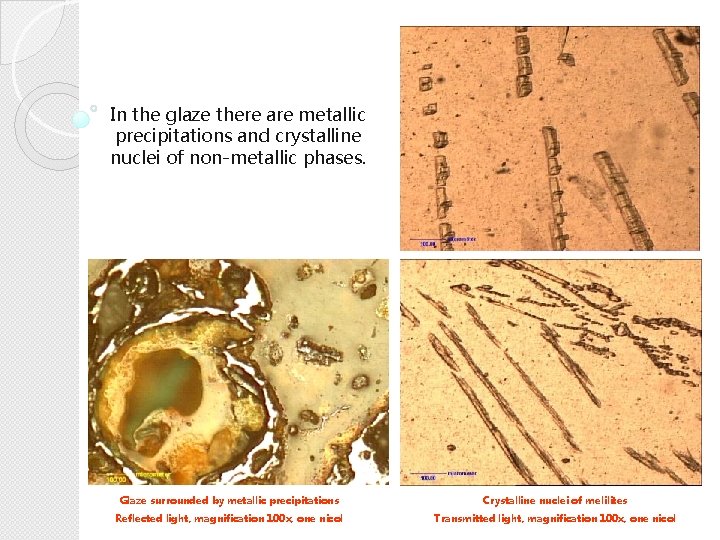
Glaze In the glaze there are metallic precipitations and crystalline nuclei of non-metallic phases. Fot. XN, 100 x Glaze surrounded by metallic precipitations Crystalline nuclei of melilites Reflected light, magnification 100 x, one nicol Transmitted light, magnification 100 x, one nicol
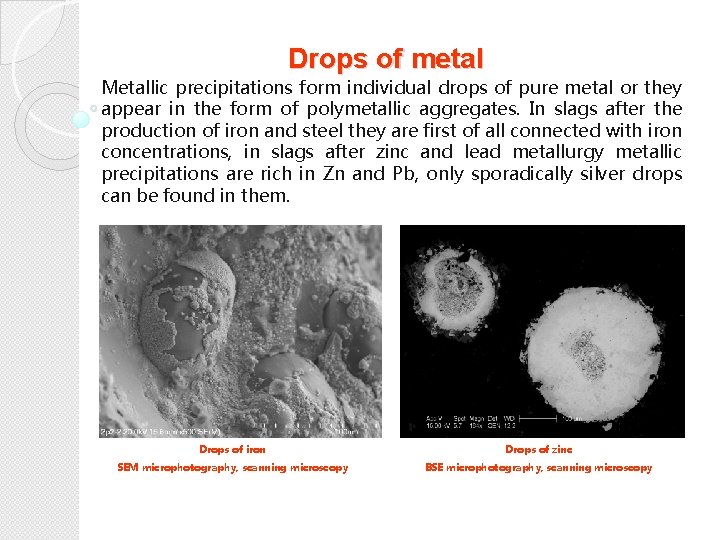
Drops of metal Metallic precipitations form individual drops of pure metal or they appear in the form of polymetallic aggregates. In slags after the production of iron and steel they are first of all connected with iron concentrations, in slags after zinc and lead metallurgy metallic precipitations are rich in Zn and Pb, only sporadically silver drops can be found in them. Drops of iron Drops of zinc SEM microphotography, scanning microscopy BSE microphotography, scanning microscopy

Metals are present in glaze, they form: inclusions in the other minerals, their own minerals (magnetite, hematite) and metallic aggregates and they are also present in the structure of other minerals (augite, mellite). Pyroxenes with inclusions of metals Quartz with cracks filled by metalic precipitations Transmitted light, magnification 200 x, one nicol Transmitted light, magnification 100 x, crossed nicols
Oxide phases identified in steelmaking slags include principally a number of iron oxides from wustite Fe. O, through magnetite Fe 3 O 4 to hematite Fe 2 O 3 and sporadic occurrence of goethite Fe. OOH.
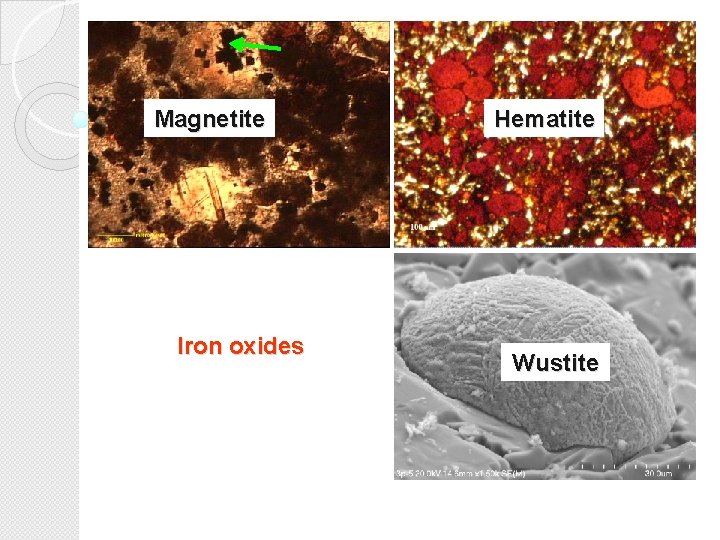
Magnetite Iron oxides Hematite Wustite
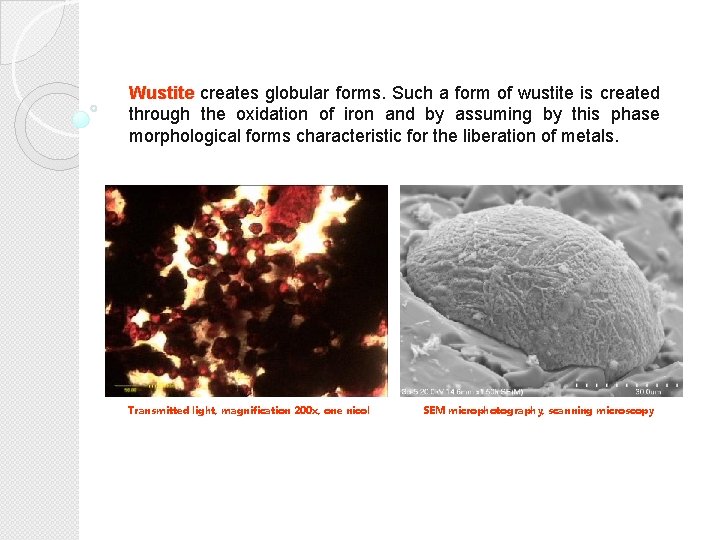
Wustite creates globular forms. Such a form of wustite is created through the oxidation of iron and by assuming by this phase morphological forms characteristic for the liberation of metals. Transmitted light, magnification 200 x, one nicol SEM microphotography, scanning microscopy

Magnetite In the waste material we can distinguish two generations of magnetite. First generation is represented by the sharp grains characterized by the square section. Its were crystallized with the other minerals during the alloy self cooling. Second generation is represented by fine grains often mixed with the other compounds of the wastes. This generation was formed as a result of devitrification of glaze or silicate phases. Transmitted light, magnification 100 x, one nicol
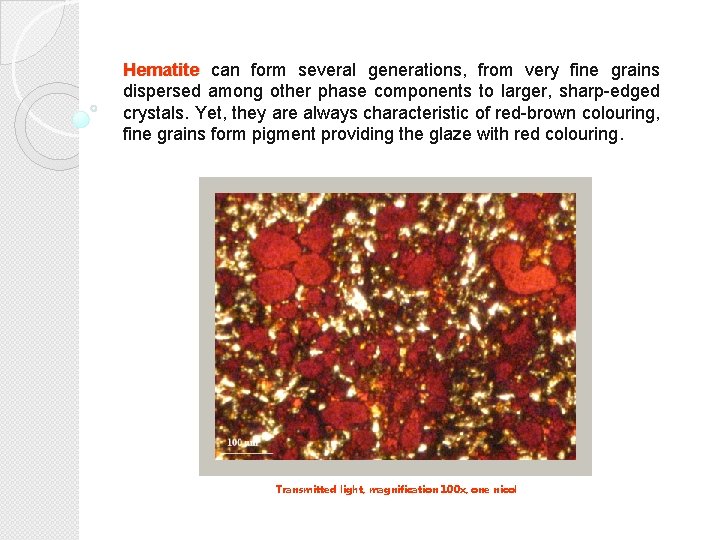
Hematite can form several generations, from very fine grains dispersed among other phase components to larger, sharp-edged crystals. Yet, they are always characteristic of red-brown colouring, fine grains form pigment providing the glaze with red colouring. Transmitted light, magnification 100 x, one nicol
- Slides: 11