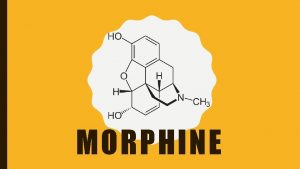
Morphine Named after the Greek God Morpheus God






















- Slides: 79


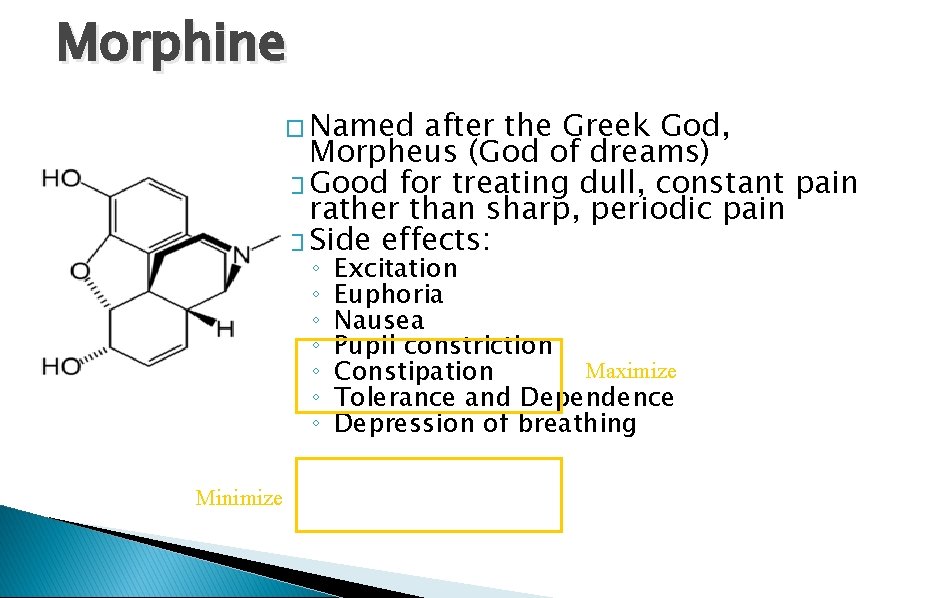
Morphine � Named after the Greek God, Morpheus (God of dreams) � Good for treating dull, constant pain rather than sharp, periodic pain � Side effects: ◦ ◦ ◦ ◦ Minimize Excitation Euphoria Nausea Pupil constriction Maximize Constipation Tolerance and Dependence Depression of breathing

- An opium alkaloid -Papaver somniferum target site: CNS SE: serious

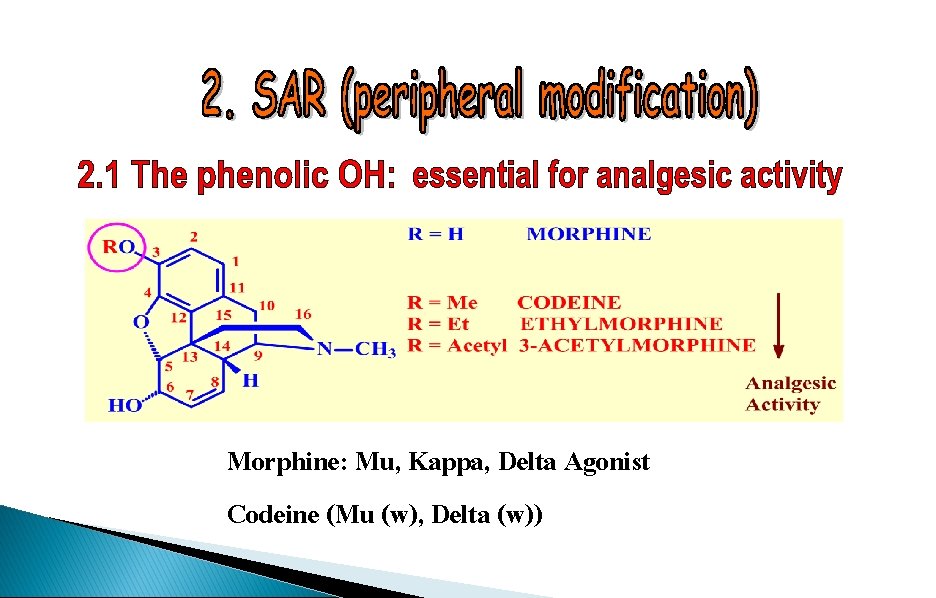
Morphine: Mu, Kappa, Delta Agonist Codeine (Mu (w), Delta (w))

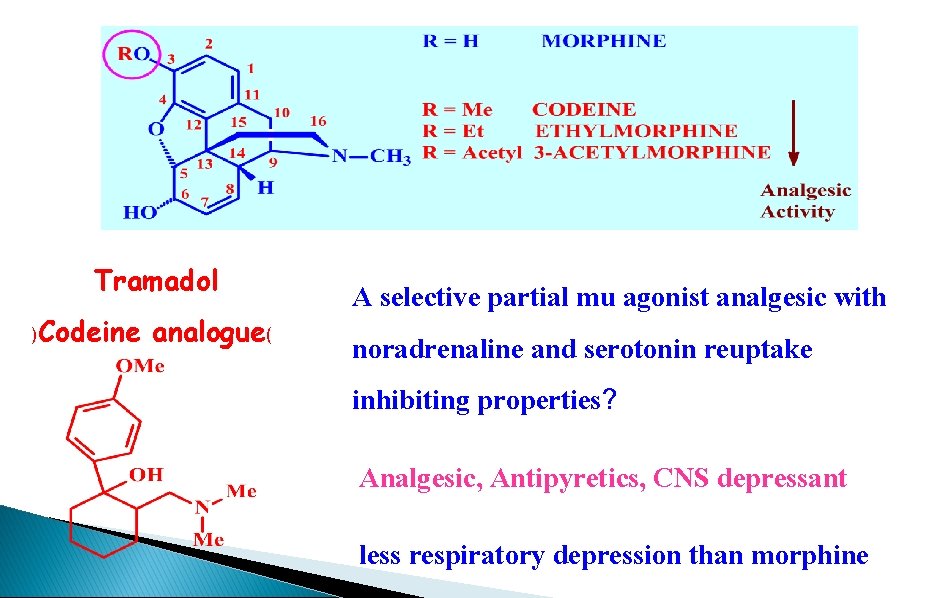
Tramadol )Codeine analogue( A selective partial mu agonist analgesic with noradrenaline and serotonin reuptake inhibiting properties? Analgesic, Antipyretics, CNS depressant less respiratory depression than morphine

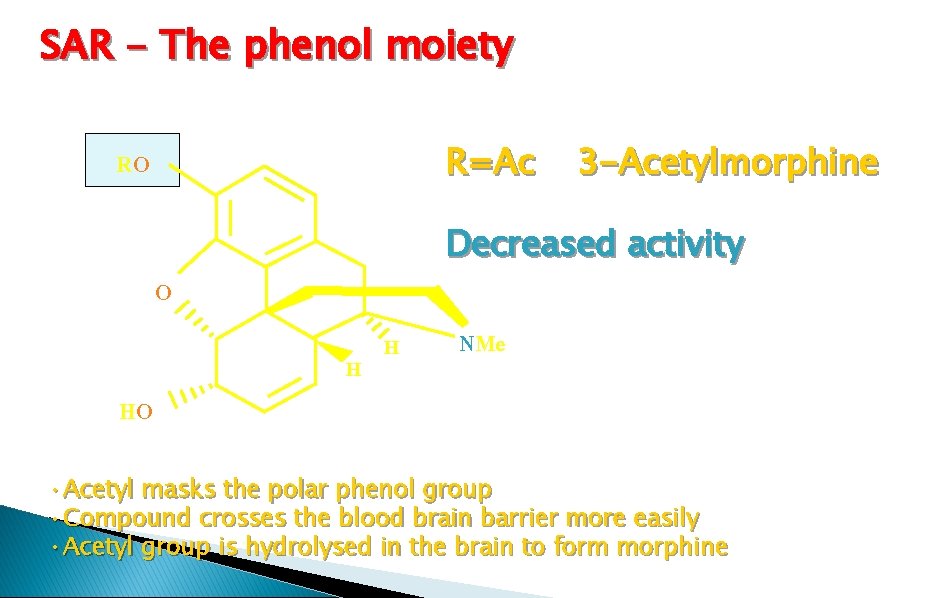
SAR - The phenol moiety Notes Codeine is metabolised in the liver to morphine. The activity observed is due to morphine. Codeine is used for mild pain and coughs Weaker analgesic but weaker side effects. Conclusion Masking phenol is bad for activity

SAR - The phenol moiety R=Ac RO Decreased activity O HO 3 -Acetylmorphine H H NMe • Acetyl masks the polar phenol group • Compound crosses the blood brain barrier more easily • Acetyl group is hydrolysed in the brain to form morphine

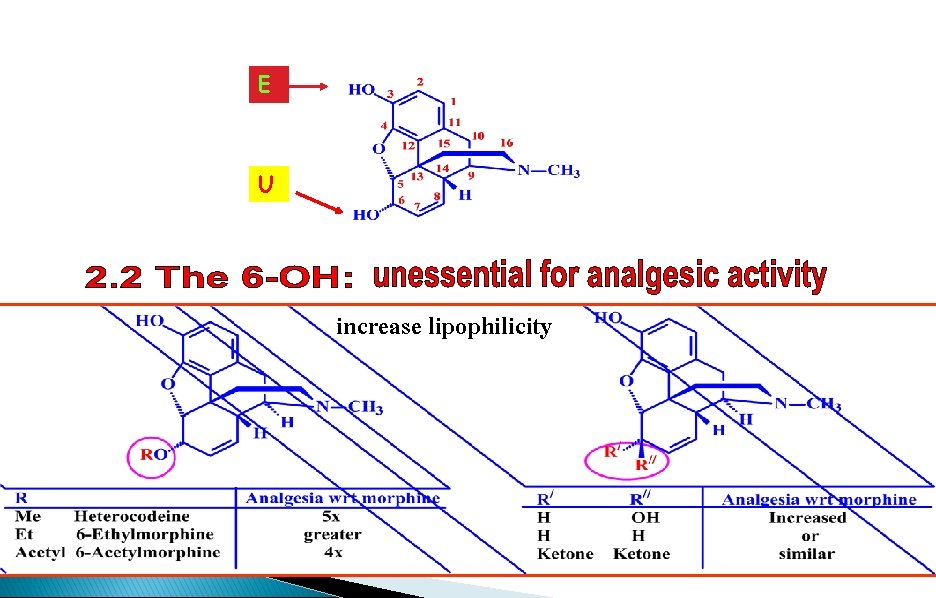
E U increase lipophilicity

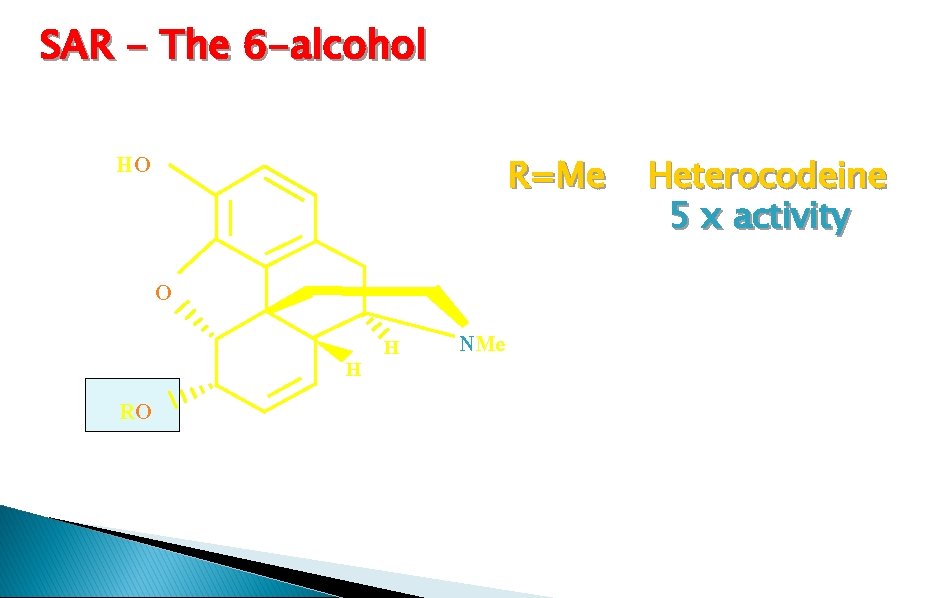
SAR - The 6 -alcohol HO R=Me O RO H H NMe Heterocodeine 5 x activity

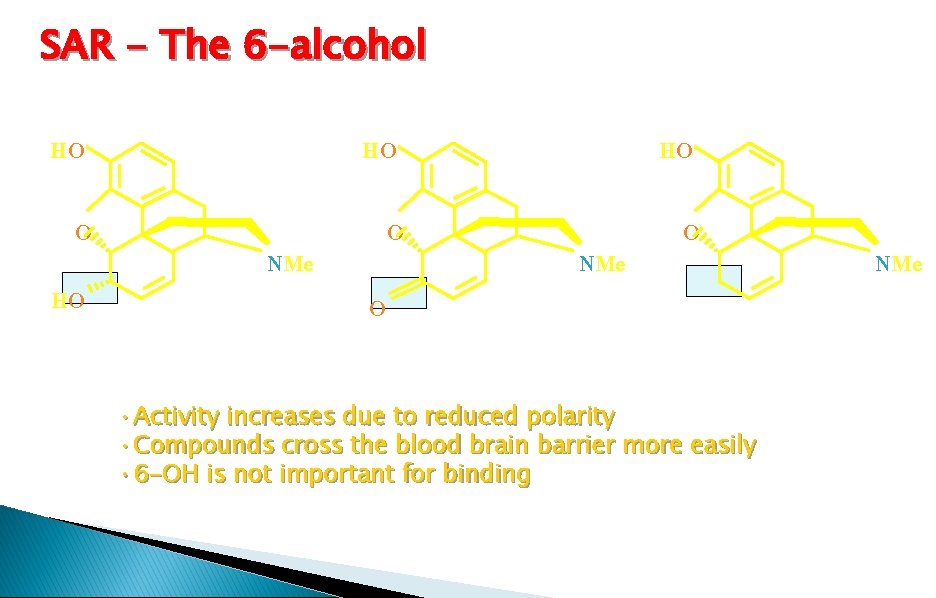
SAR - The 6 -alcohol HO HO HO NMe • Activity increases due to reduced polarity • Compounds cross the blood brain barrier more easily • 6 -OH is not important for binding NMe

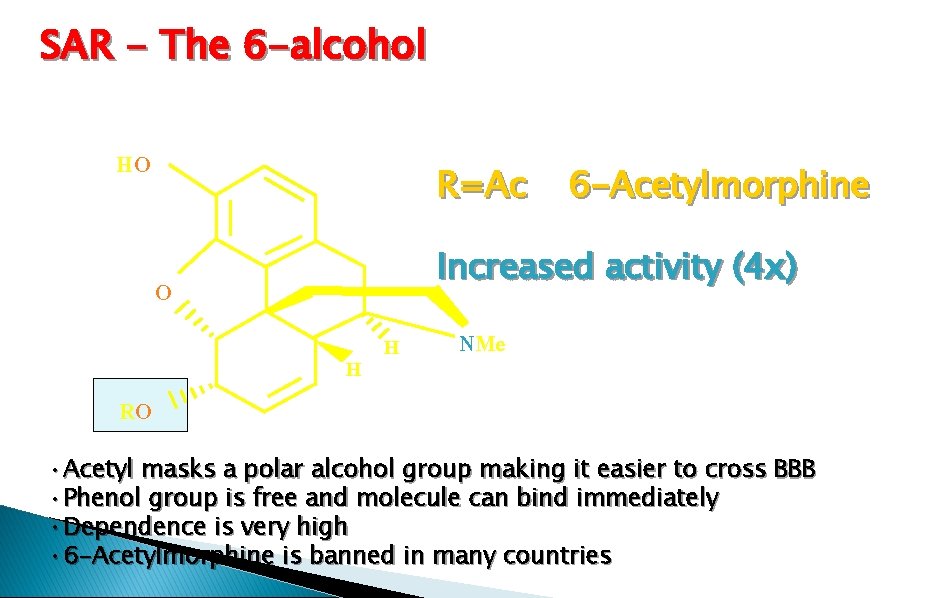
SAR - The 6 -alcohol HO R=Ac Increased activity (4 x) O RO 6 -Acetylmorphine H H NMe • Acetyl masks a polar alcohol group making it easier to cross BBB • Phenol group is free and molecule can bind immediately • Dependence is very high • 6 -Acetylmorphine is banned in many countries

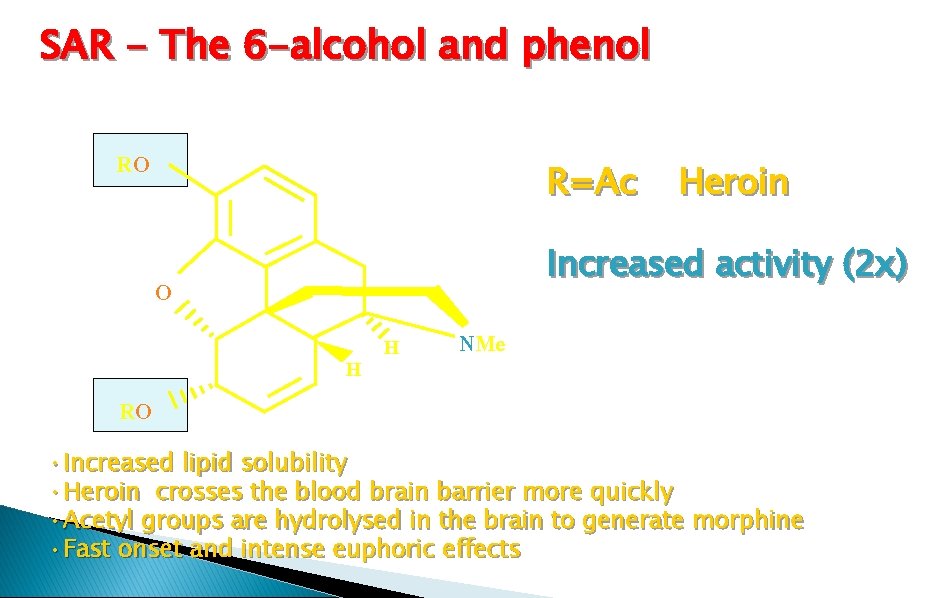
SAR - The 6 -alcohol and phenol RO R=Ac Increased activity (2 x) O RO Heroin H H NMe • Increased lipid solubility • Heroin crosses the blood brain barrier more quickly • Acetyl groups are hydrolysed in the brain to generate morphine • Fast onset and intense euphoric effects

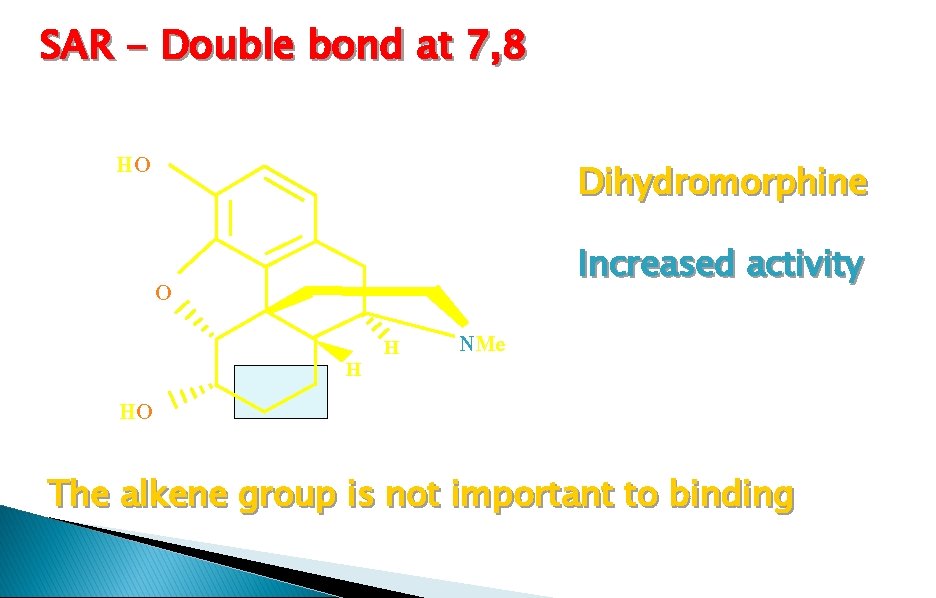
SAR - Double bond at 7, 8 HO Dihydromorphine Increased activity O HO H H NMe The alkene group is not important to binding

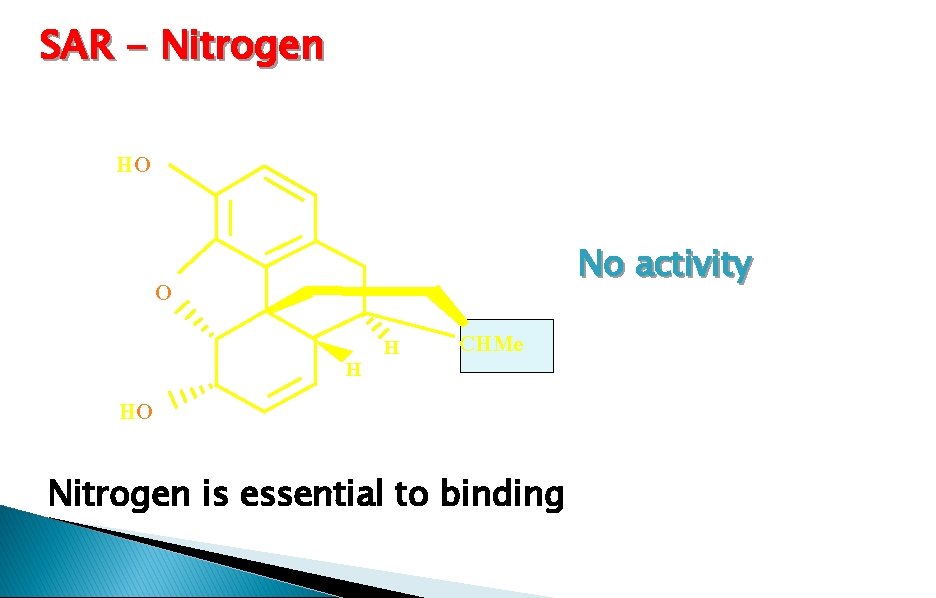
SAR - Nitrogen HO No activity O HO H H CHMe Nitrogen is essential to binding

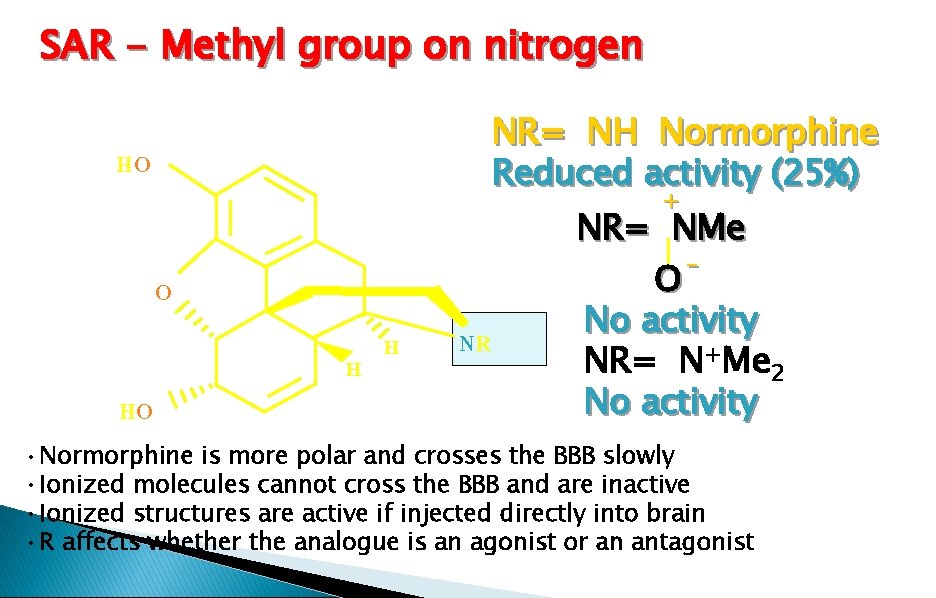
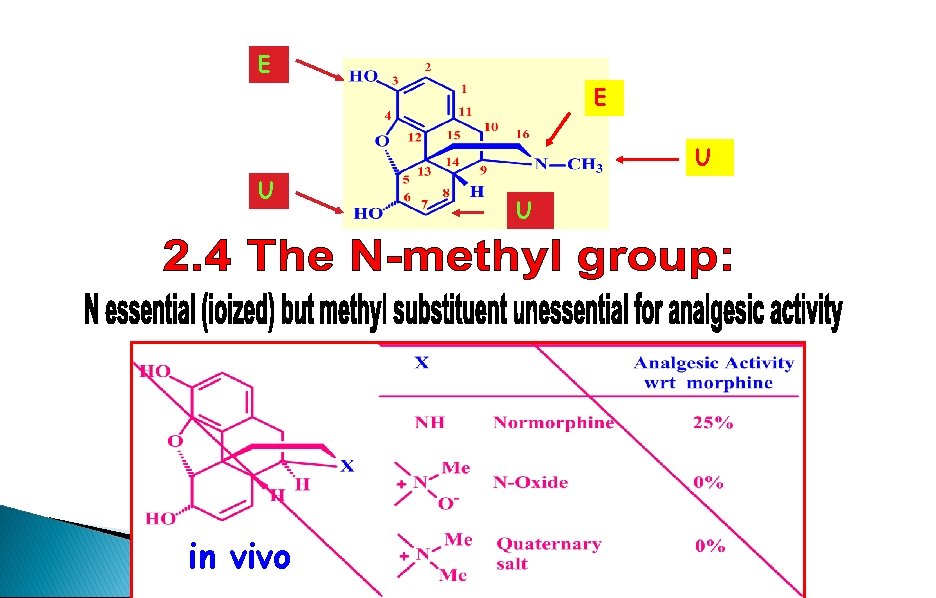
SAR - Methyl group on nitrogen HO NR= NH Normorphine Reduced activity (25%) HO NR= NMe O No activity NR= N+Me 2 No activity + O H H NR • Normorphine is more polar and crosses the BBB slowly • Ionized molecules cannot cross the BBB and are inactive • Ionized structures are active if injected directly into brain • R affects whether the analogue is an agonist or an antagonist

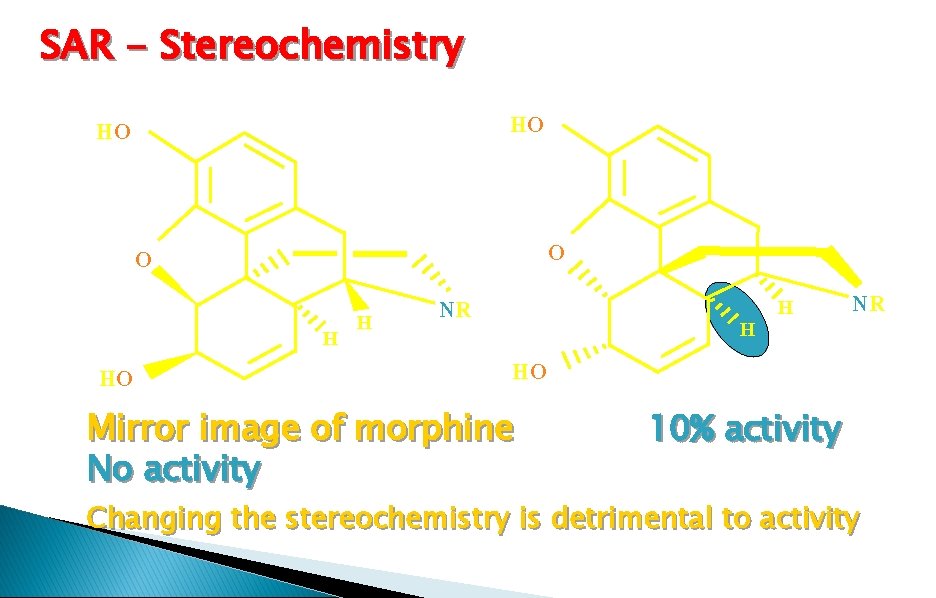
SAR - Stereochemistry HO HO O O HO H H NR HO Mirror image of morphine No activity H H NR 10% activity Changing the stereochemistry is detrimental to activity

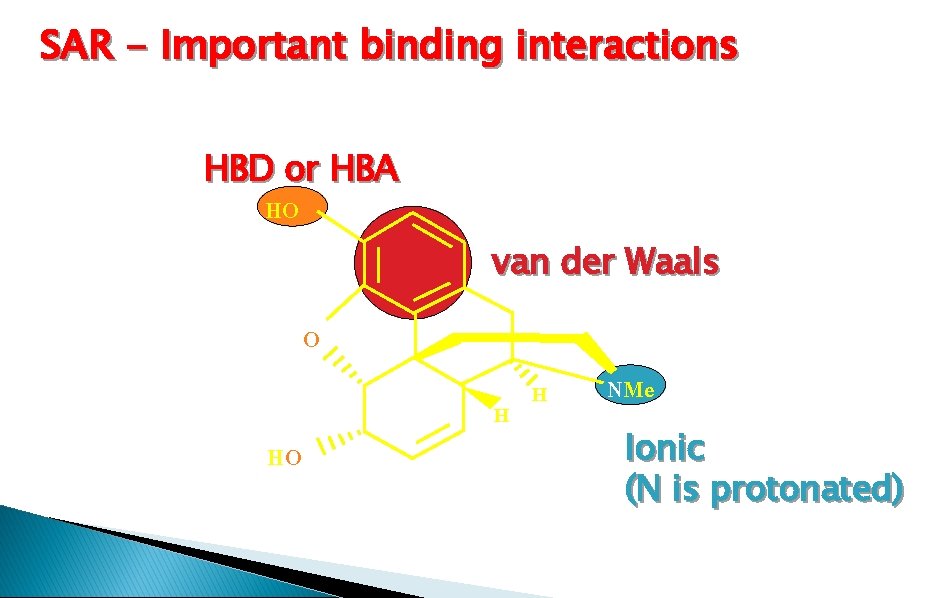
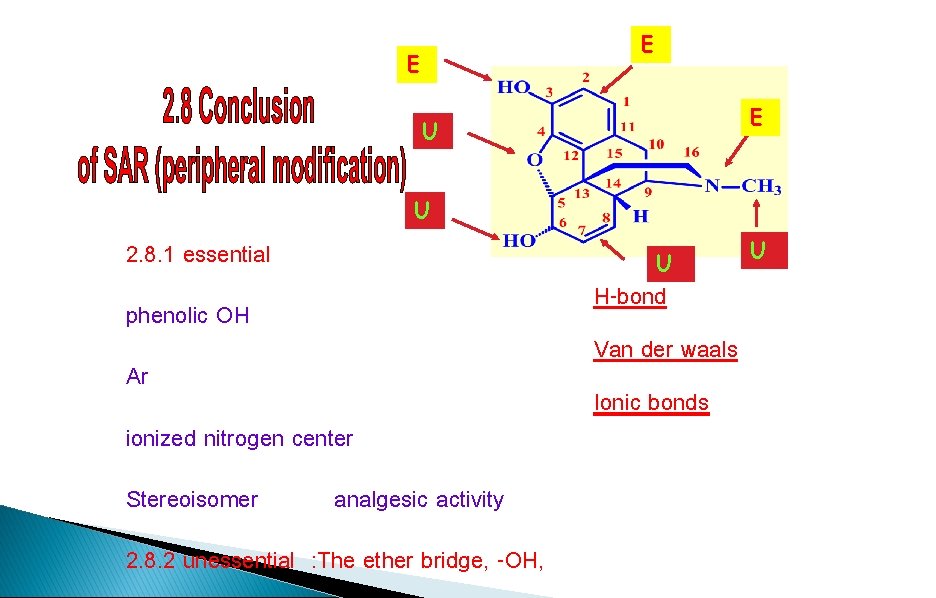
SAR - Important binding interactions HBD or HBA HO van der Waals O HO H H NMe Ionic (N is protonated)

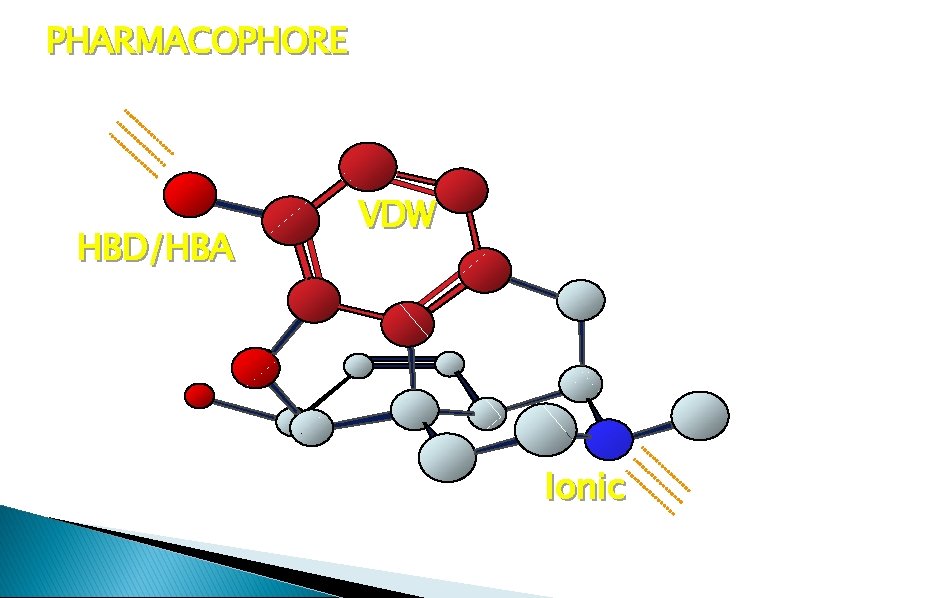
PHARMACOPHORE HBD/HBA VDW Ionic

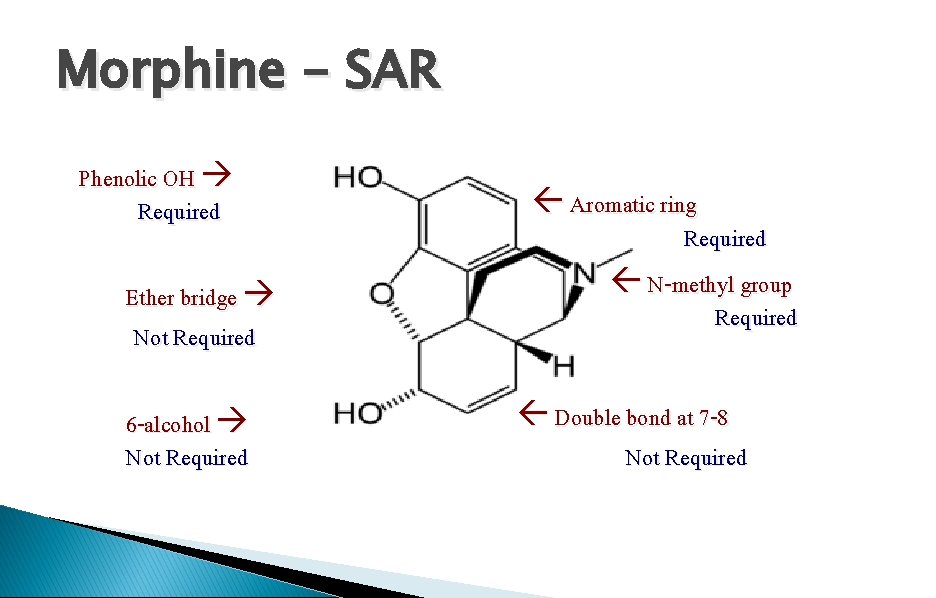
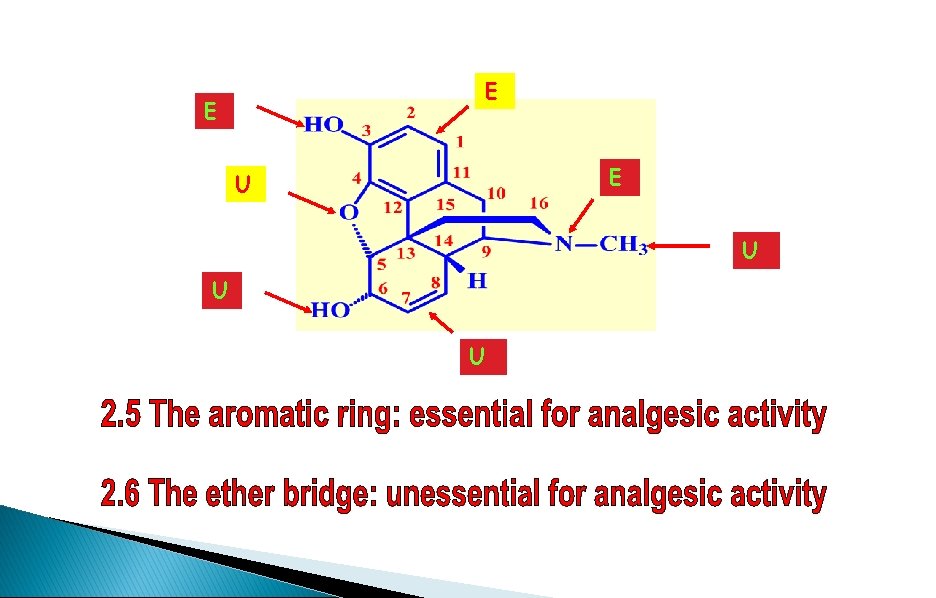
Morphine - SAR Phenolic OH Required Ether bridge Not Required 6 -alcohol Not Required Aromatic ring Required N-methyl group Required Double bond at 7 -8 Not Required

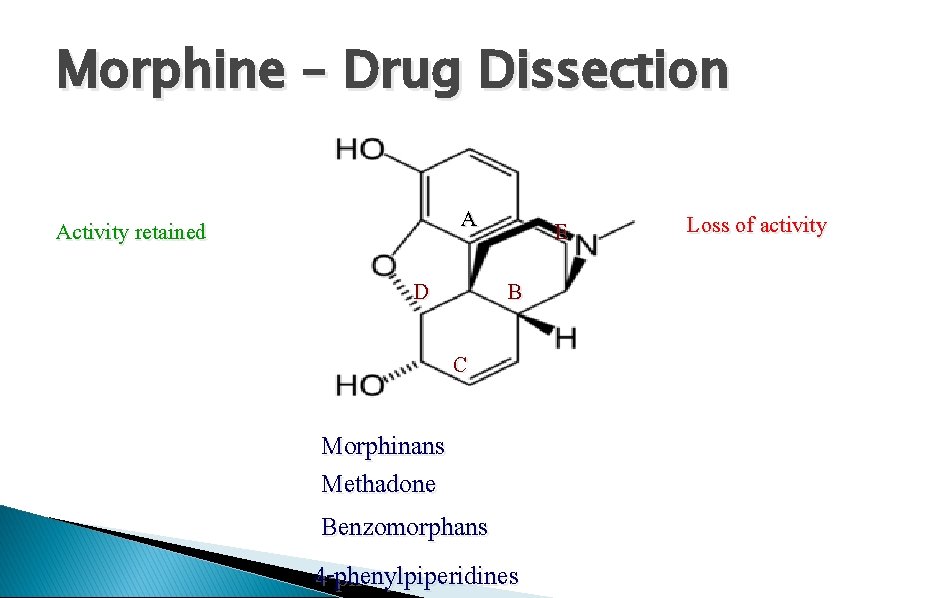
Morphine – Drug Dissection A Activity retained D E B C Morphinans Methadone Benzomorphans 4 -phenylpiperidines Loss of activity

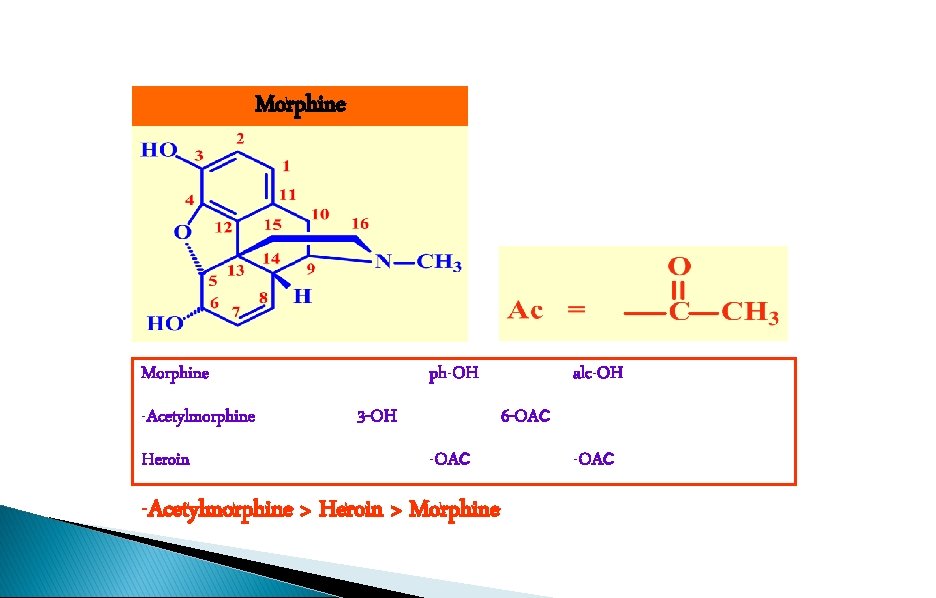
Morphine -Acetylmorphine Heroin ph-OH 3 -OH alc-OH 6 -OAc -Acetylmorphine > Heroin > Morphine -OAc

E U U

E E U U in vivo U

E E E U U


E E E U U 2. 8. 1 essential U H-bond phenolic OH Van der waals Ar ionized nitrogen center Stereoisomer analgesic activity 2. 8. 2 unessential : The ether bridge, -OH, Ionic bonds U

morphine codeine 6 - acetylmorphine heroin hydromorphone hydrocodone oxymorphone oxycodone

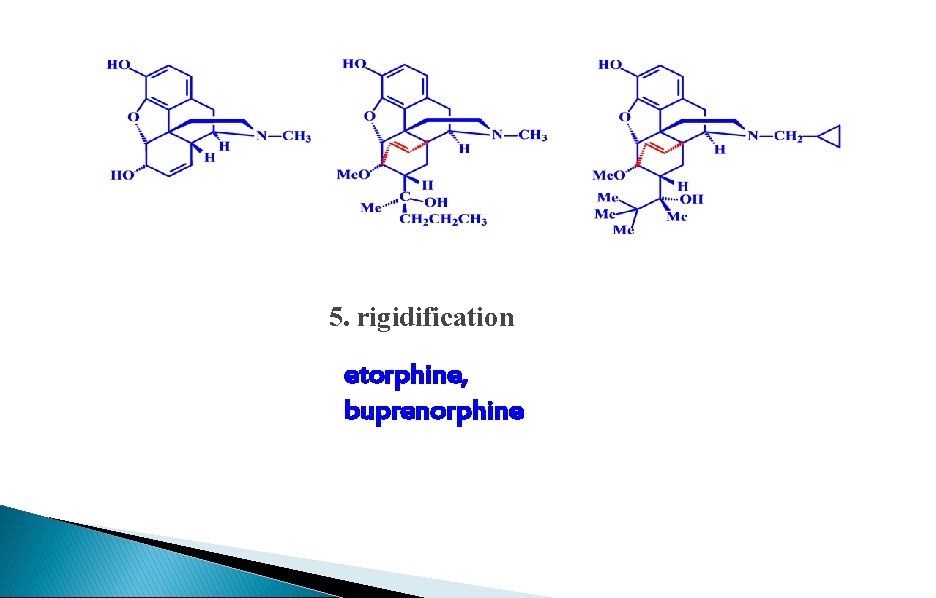
- drug extension - simplification - rigidification

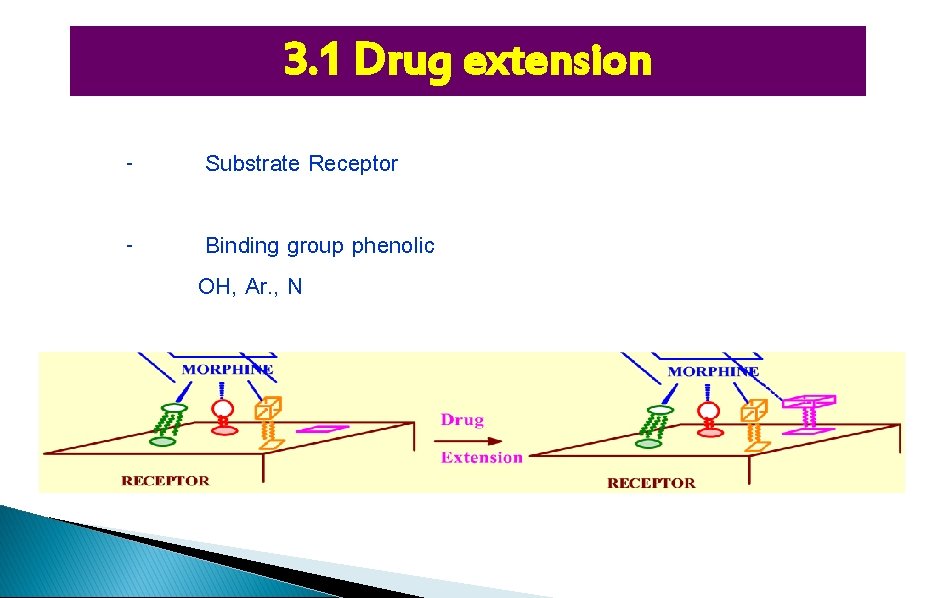
3. 1 Drug extension - Substrate Receptor - Binding group phenolic OH, Ar. , N

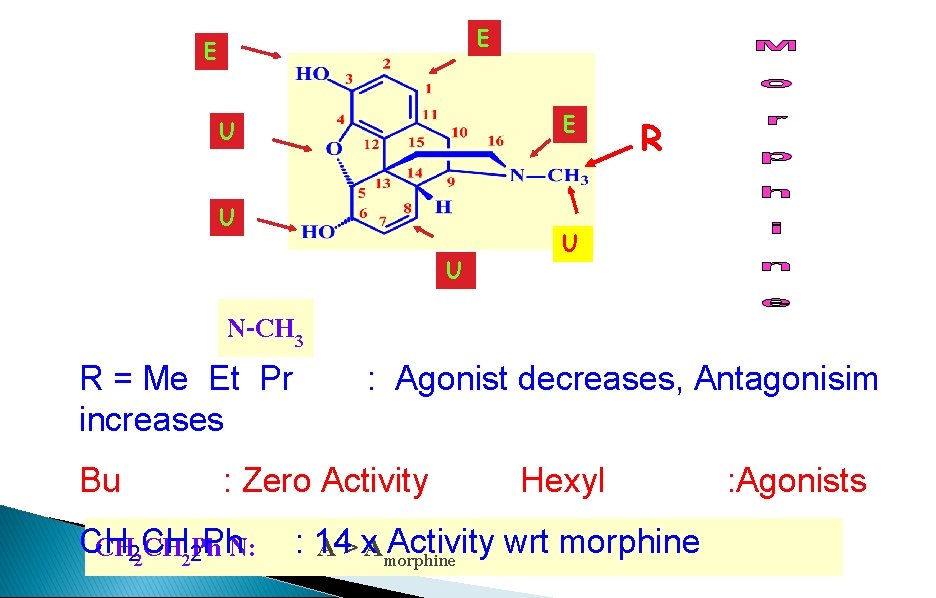
E E E U U U R U N-CH 3 R = Me Et Pr increases Bu : Agonist decreases, Antagonisim : Zero Activity CH CH 22 Ph. N: Hexyl : 14 Activity wrt morphine A > x. Amorphine : Agonists

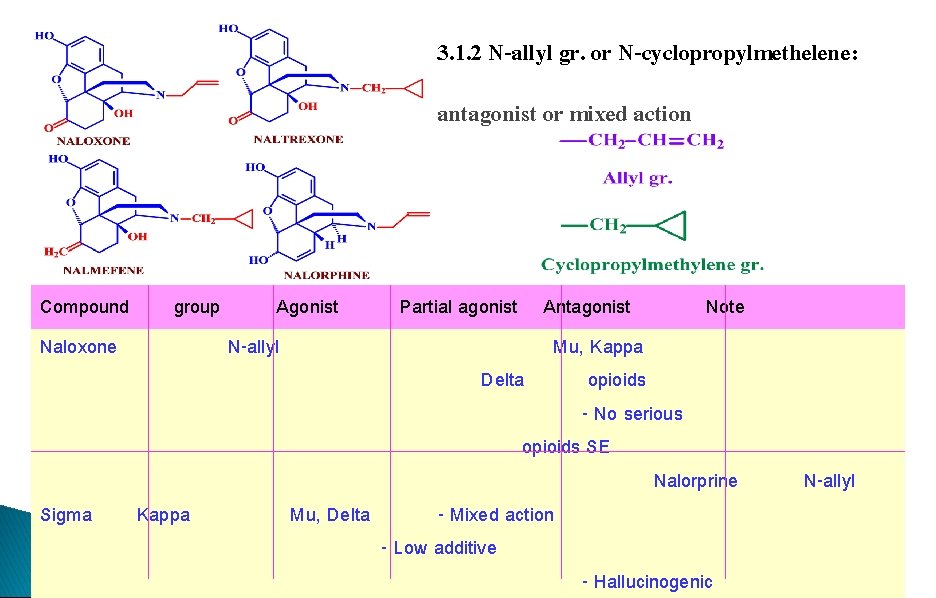
3. 1. 2 N-allyl gr. or N-cyclopropylmethelene: antagonist or mixed action Compound Naloxone Sigma group Kappa Agonist N-allyl Partial agonist Antagonist Note Mu, Kappa Delta opioids - No serious opioids SE Nalorprine Mu, Delta - Mixed action - Low additive - Hallucinogenic N-allyl

Naloxone Naltrexon e Nalmefen e

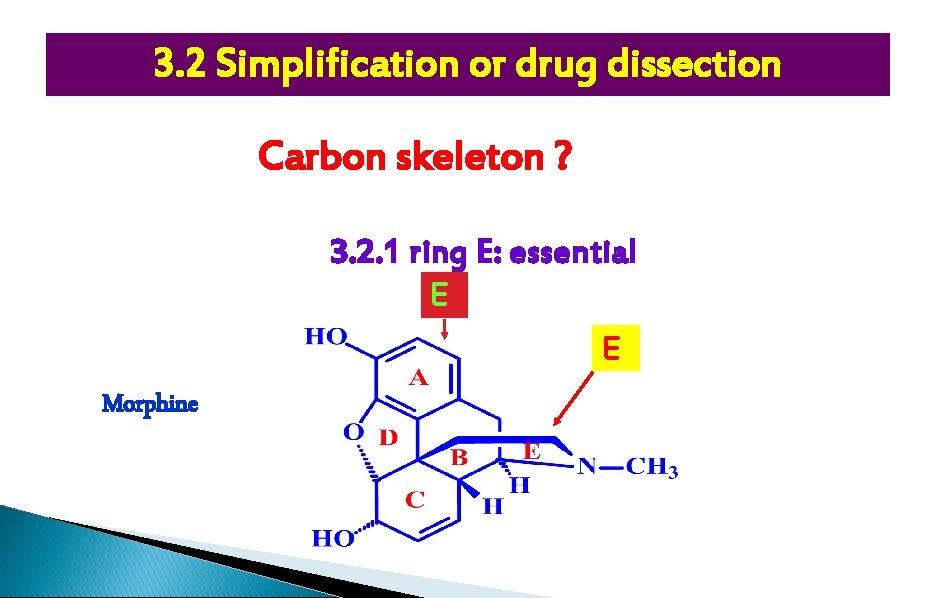
3. 2 Simplification or drug dissection Carbon skeleton ? 3. 2. 1 ring E: essential E E Morphine

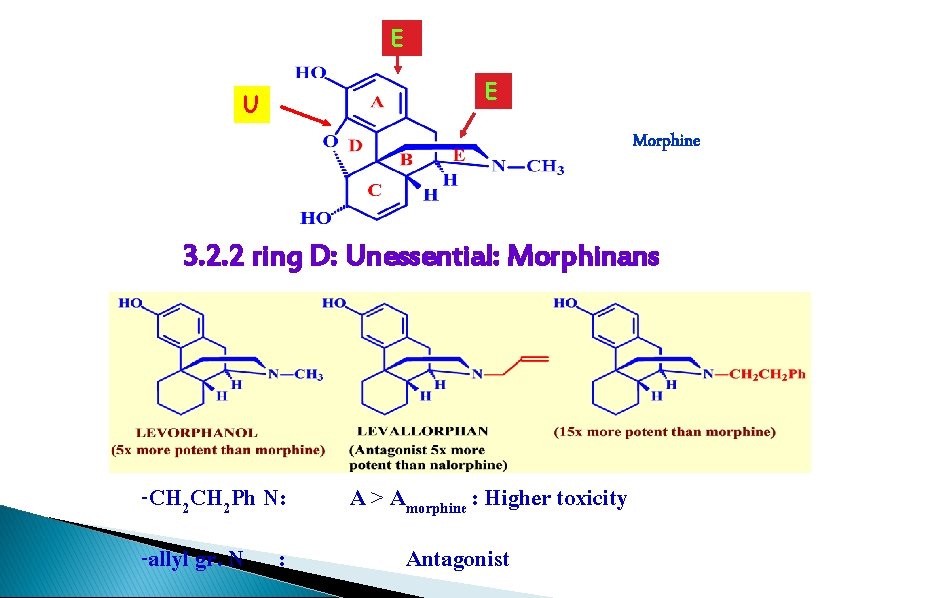
E U E Morphine 3. 2. 2 ring D: Unessential: Morphinans -CH 2 Ph N: -allyl gr. N : A > Amorphine : Higher toxicity Antagonist

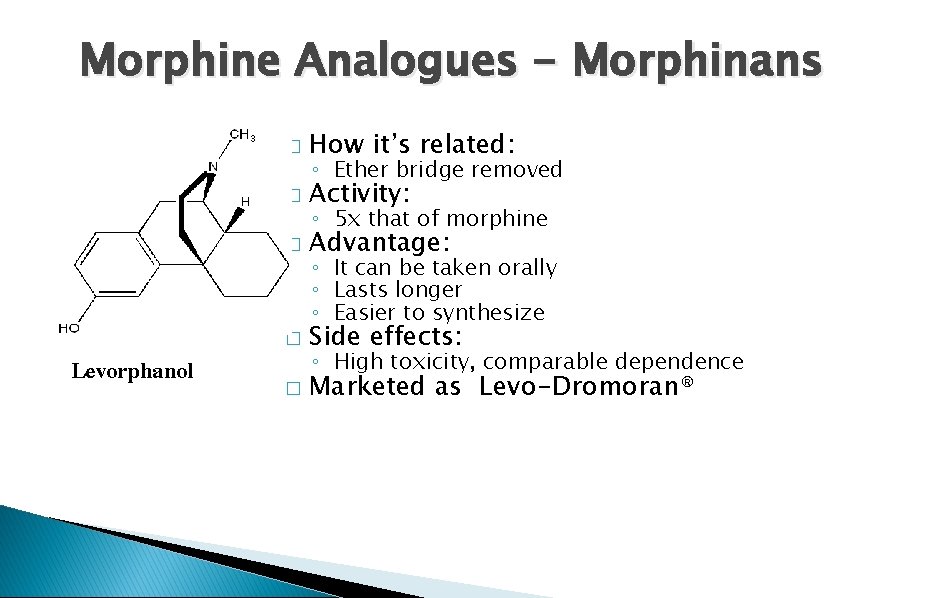
Morphine Analogues - Morphinans Levorphanol � How it’s related: � Activity: � Advantage: � Side effects: � Marketed as Levo-Dromoran® ◦ Ether bridge removed ◦ 5 x that of morphine ◦ It can be taken orally ◦ Lasts longer ◦ Easier to synthesize ◦ High toxicity, comparable dependence

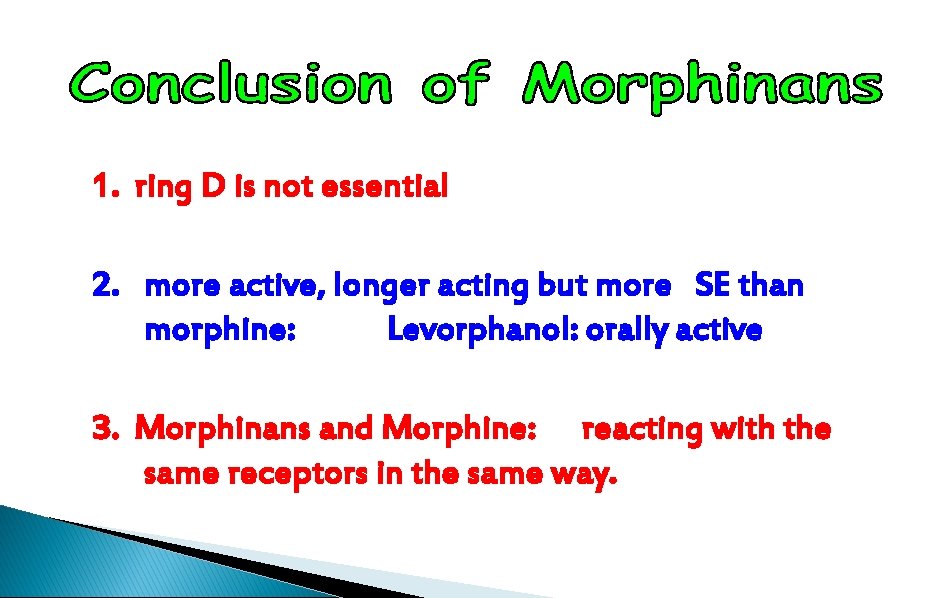
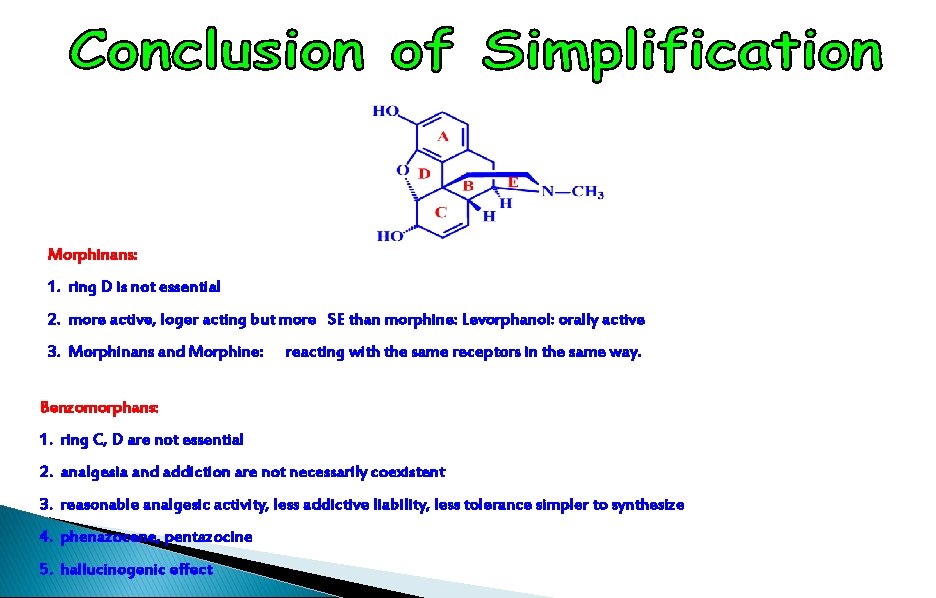
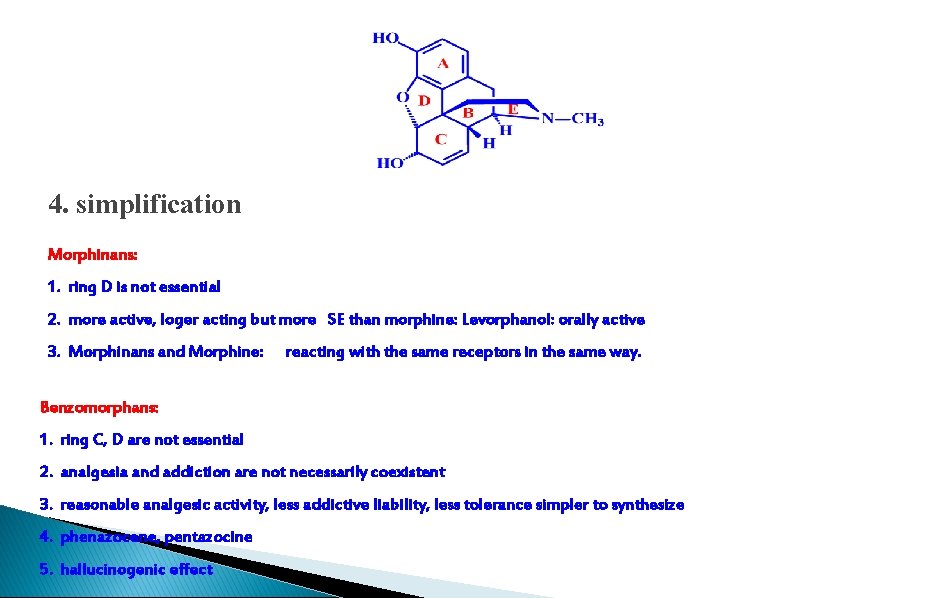
1. ring D is not essential 2. more active, longer acting but more SE than morphine: Levorphanol: orally active 3. Morphinans and Morphine: reacting with the same receptors in the same way.

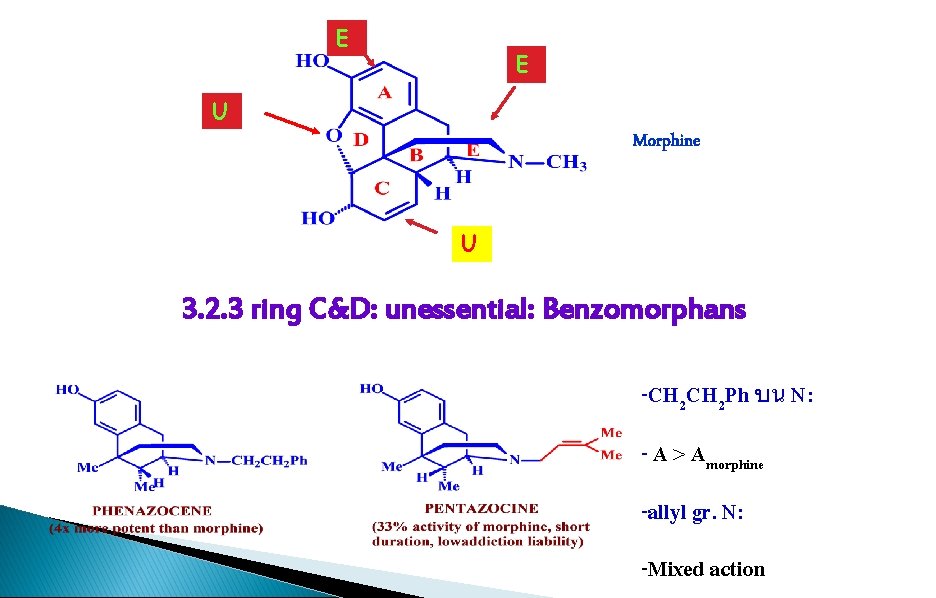
E E U Morphine U 3. 2. 3 ring C&D: unessential: Benzomorphans -CH 2 Ph บน N: - A > Amorphine -allyl gr. N: -Mixed action

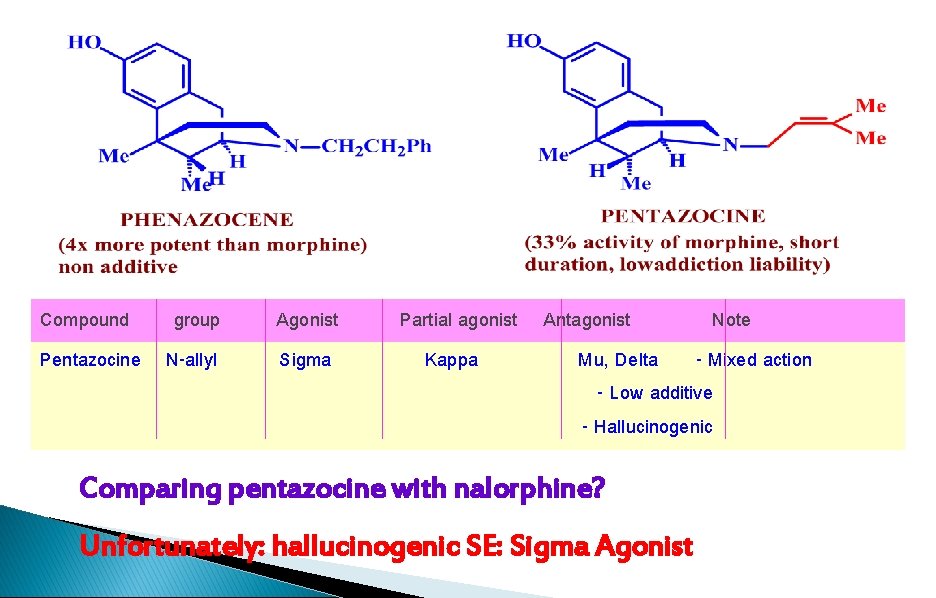
Compound group Pentazocine N-allyl Agonist Sigma Partial agonist Antagonist Note Kappa Mu, Delta - Mixed action - Low additive - Hallucinogenic Comparing pentazocine with nalorphine? Unfortunately: hallucinogenic SE: Sigma Agonist

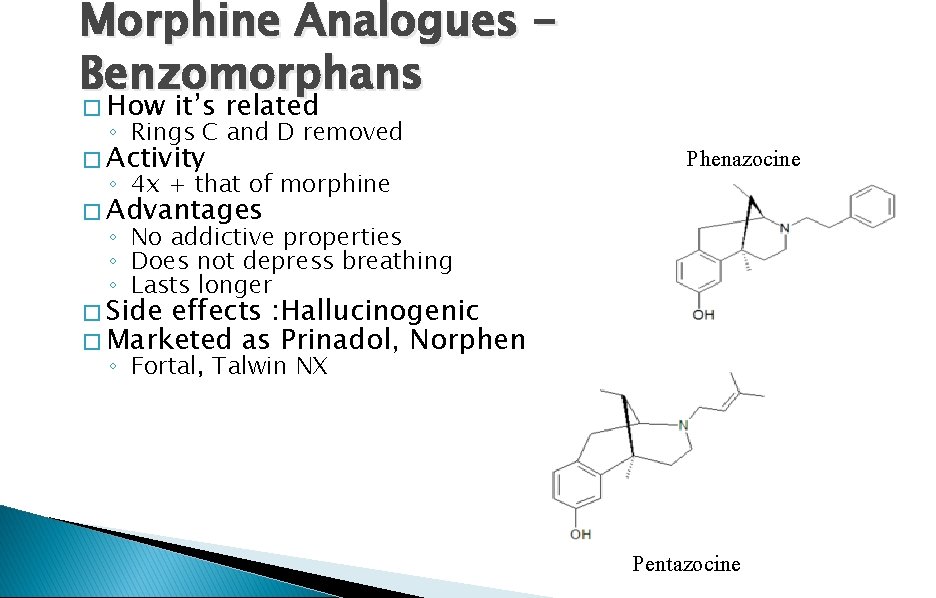
Morphine Analogues Benzomorphans � How it’s related ◦ Rings C and D removed � Activity ◦ 4 x + that of morphine Phenazocine � Advantages ◦ No addictive properties ◦ Does not depress breathing ◦ Lasts longer � Side effects : Hallucinogenic � Marketed as Prinadol, Norphen ◦ Fortal, Talwin NX Pentazocine

1. ring C, D are not essential 2. analgesia and addiction are not necessarily coexistent 3. reasonable analgesic activity, less addictive liability, less tolerance 4. simpler to synthesize 5. phenazocene, pentazocine 6. hallucinogenic effect

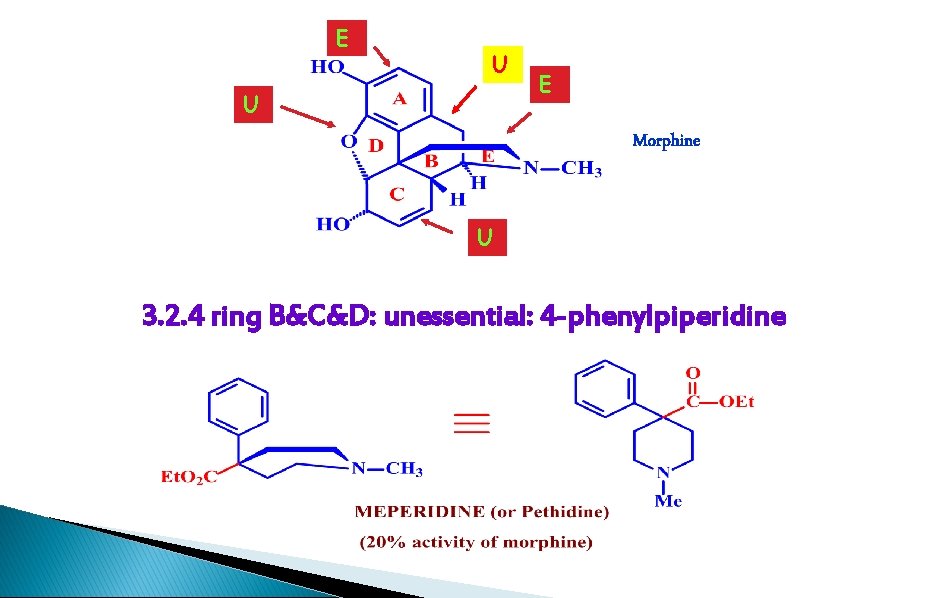
E U U E Morphine U 3. 2. 4 ring B&C&D: unessential: 4 -phenylpiperidine

Morphine more potent than morphin e because of more lipophilici ty

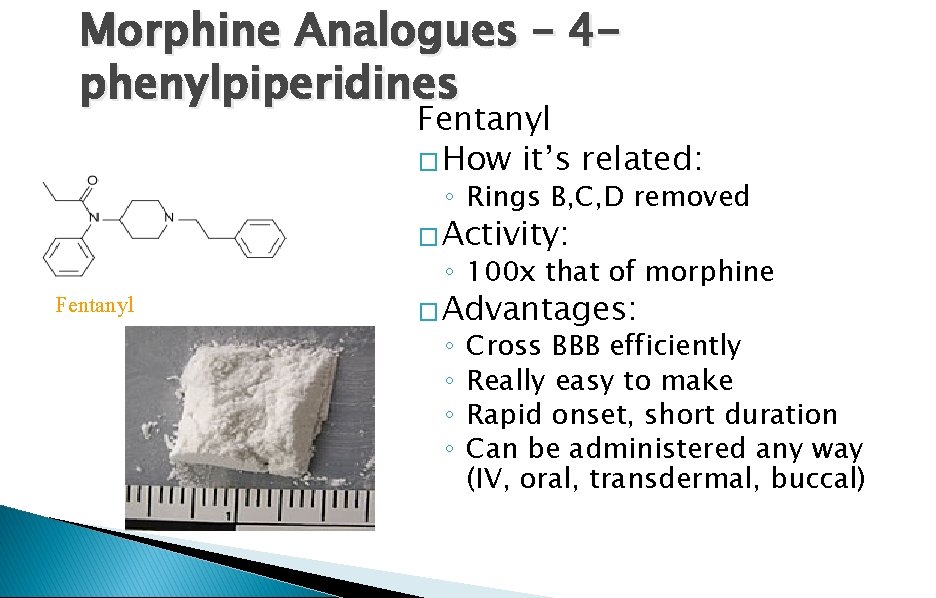
Morphine Analogues – 4 phenylpiperidines Fentanyl � How it’s related: ◦ Rings B, C, D removed � Activity: Fentanyl ◦ 100 x that of morphine � Advantages: ◦ ◦ Cross BBB efficiently Really easy to make Rapid onset, short duration Can be administered any way (IV, oral, transdermal, buccal)

Morphine Analogues – 4 phenylpiperidines � Used for: ◦ Anesthesia ◦ Chronic pain management � Side effects: ◦ Sudden respiratory depression ◦ More addictive than heroin ◦ Less euphoria, more sedation � Marketed as: ◦ Sufenta (used in ♥ surgery) ◦ Carfentanil (used in vet practice) ◦ “Percopop”, Oxy. Contin, “magic” (heroin/cocaine)


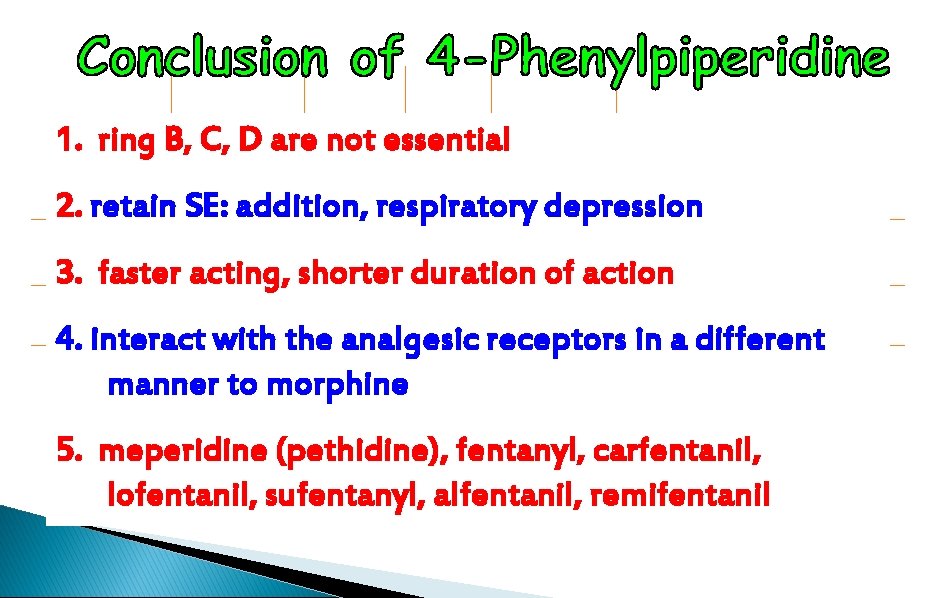
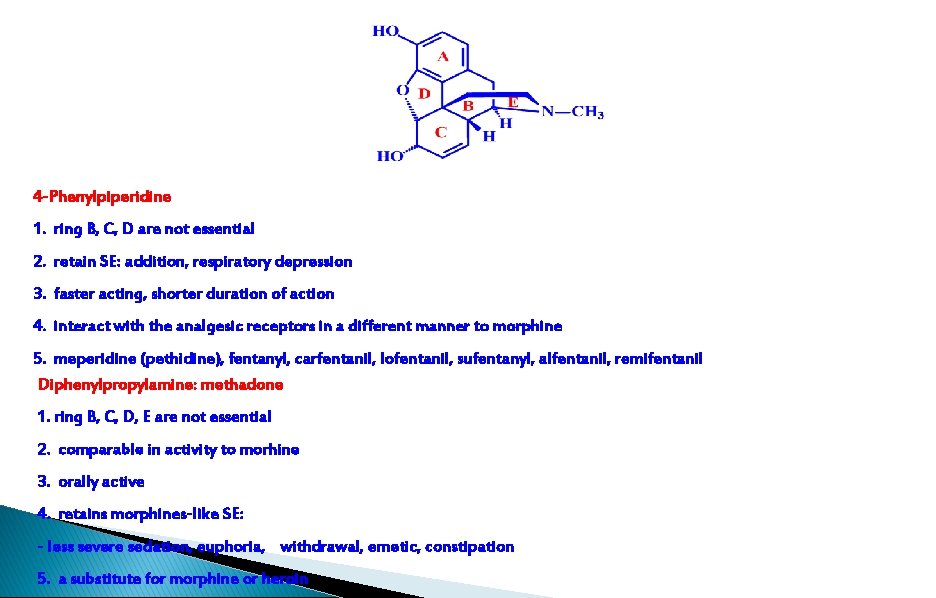
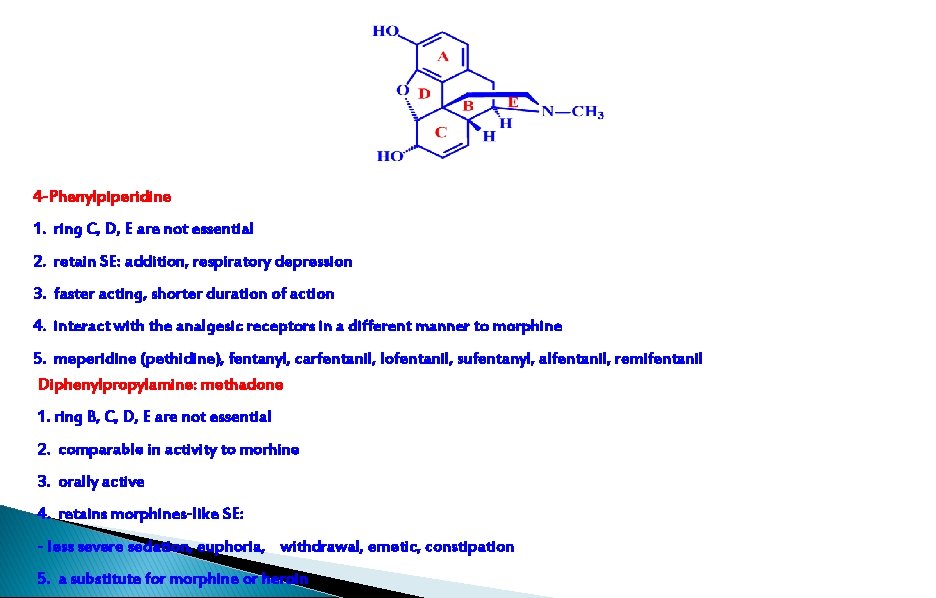
1. ring B, C, D are not essential 2. retain SE: addition, respiratory depression 3. faster acting, shorter duration of action 4. interact with the analgesic receptors in a different manner to morphine 5. meperidine (pethidine), fentanyl, carfentanil, lofentanil, sufentanyl, alfentanil, remifentanil

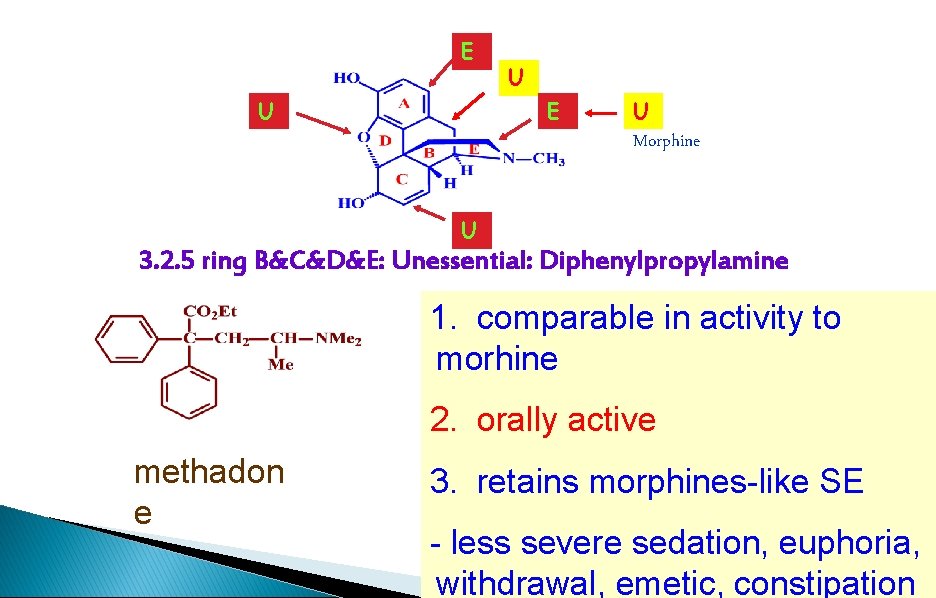
E U U E U Morphine U 3. 2. 5 ring B&C&D&E: Unessential: Diphenylpropylamine 1. comparable in activity to morhine 2. orally active methadon e 3. retains morphines-like SE - less severe sedation, euphoria, withdrawal, emetic, constipation

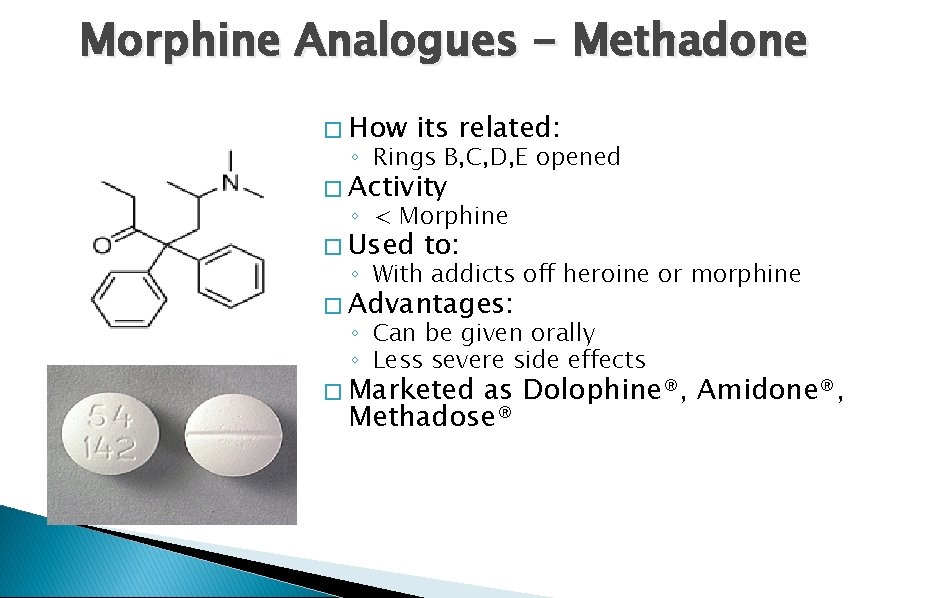
Morphine Analogues - Methadone � How its related: ◦ Rings B, C, D, E opened � Activity ◦ < Morphine � Used to: ◦ With addicts off heroine or morphine � Advantages: ◦ Can be given orally ◦ Less severe side effects � Marketed as Dolophine®, Amidone®, Methadose®

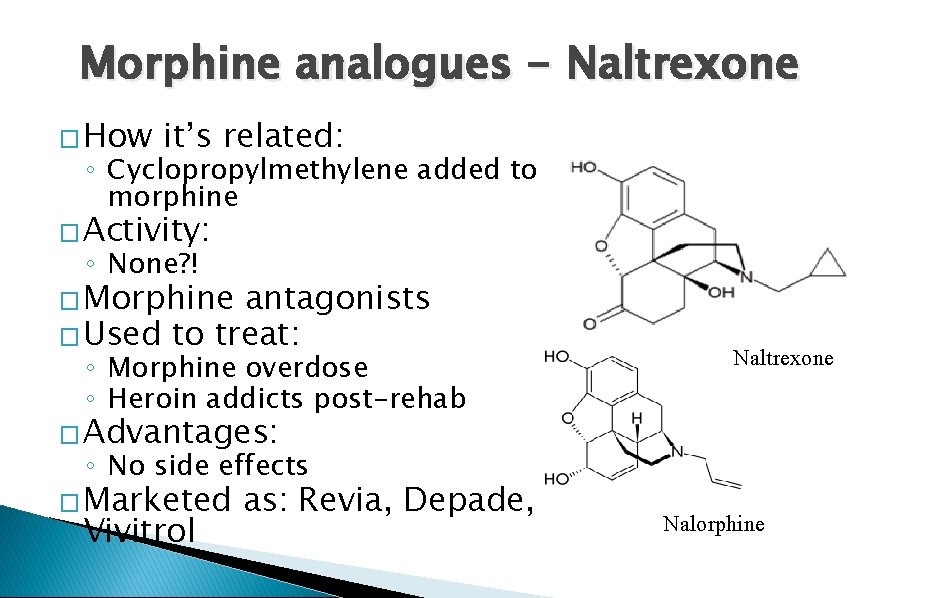
Morphine analogues - Naltrexone � How it’s related: ◦ Cyclopropylmethylene added to morphine � Activity: ◦ None? ! � Morphine antagonists � Used to treat: ◦ Morphine overdose ◦ Heroin addicts post-rehab Naltrexone � Advantages: ◦ No side effects � Marketed Vivitrol as: Revia, Depade, Nalorphine

Morphinans: 1. ring D is not essential 2. more active, loger acting but more SE than morphine: Levorphanol: orally active 3. Morphinans and Morphine: reacting with the same receptors in the same way. Benzomorphans: 1. ring C, D are not essential 2. analgesia and addiction are not necessarily coexistent 3. reasonable analgesic activity, less addictive liability, less tolerance simpler to synthesize 4. phenazocene, pentazocine 5. hallucinogenic effect

4 -Phenylpiperidine 1. ring B, C, D are not essential 2. retain SE: addition, respiratory depression 3. faster acting, shorter duration of action 4. interact with the analgesic receptors in a different manner to morphine 5. meperidine (pethidine), fentanyl, carfentanil, lofentanil, sufentanyl, alfentanil, remifentanil Diphenylpropylamine: methadone 1. ring B, C, D, E are not essential 2. comparable in activity to morhine 3. orally active 4. retains morphines-like SE: - less severe sedation, euphoria, withdrawal, emetic, constipation 5. a substitute for morphine or heroin

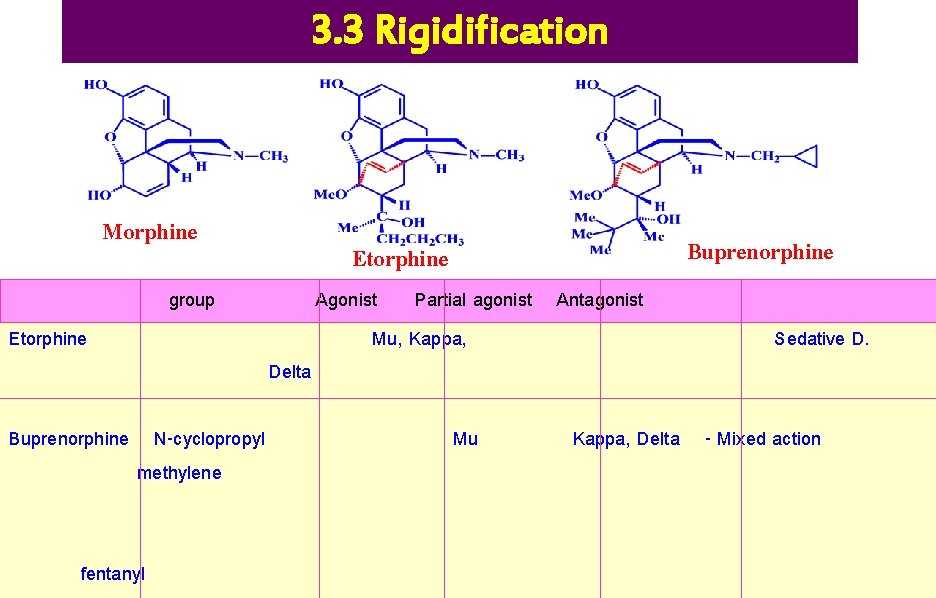
3. 3 Rigidification Morphine group Etorphine Buprenorphine N-cyclopropyl methylene fentanyl Buprenorphine Etorphine Delta Agonist Partial agonist Antagonist Mu, Kappa, Mu Sedative D. Kappa, Delta - Mixed action

Opioids receptors. 1. Mu: Mu 1: Mu 2: . 2. Kappa: , Sedation -Morphine -Nalorprine -Pentazocine -Buprenorphine 3. Delta: + ? Non-opioids receptors Sigma: + Psychotomimetic: Hallucinogenic effect

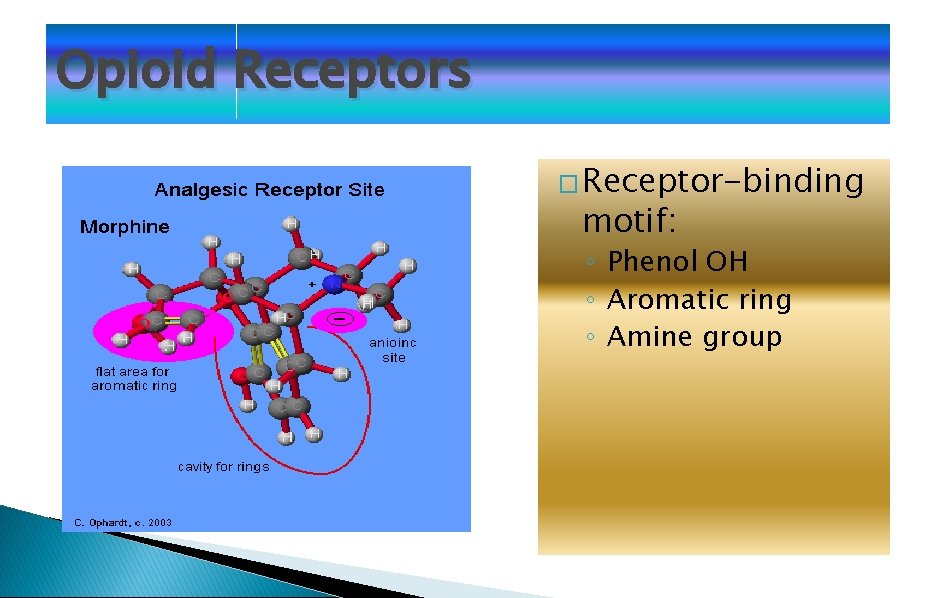
Opioid Receptors � Receptor-binding motif: ◦ Phenol OH ◦ Aromatic ring ◦ Amine group

An ideal analgesic agent? Orally active Mu: antagonist Kappa: agonist Sigma: No activity

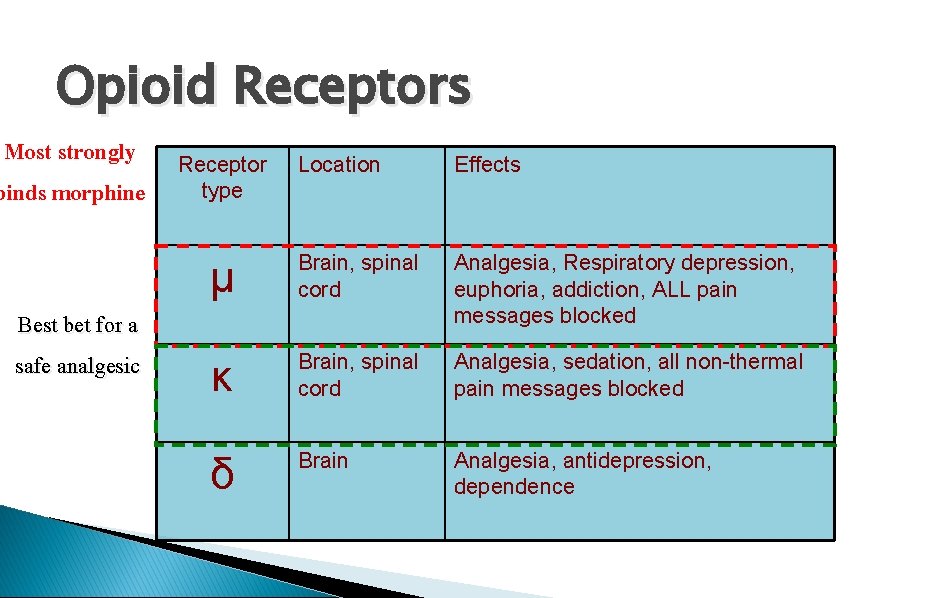
Opioid Receptors Most strongly binds morphine Best bet for a safe analgesic Receptor type Location Effects μ Brain, spinal cord Analgesia, Respiratory depression, euphoria, addiction, ALL pain messages blocked κ Brain, spinal cord Analgesia, sedation, all non-thermal pain messages blocked δ Brain Analgesia, antidepression, dependence

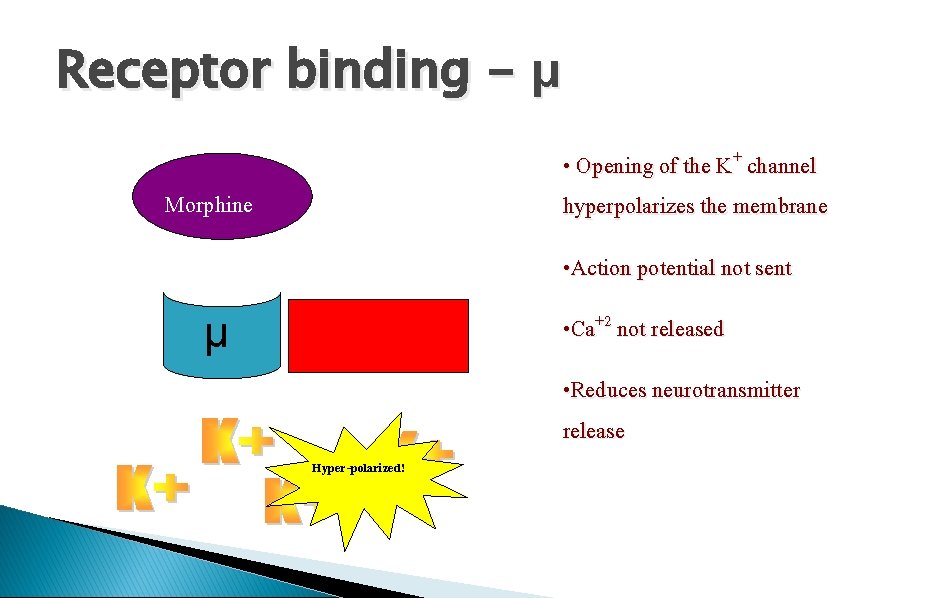
Receptor binding - μ • Opening of the K+ channel hyperpolarizes the membrane Morphine • Action potential not sent • Ca+2 not released μ • Reduces neurotransmitter release Hyper-polarized!

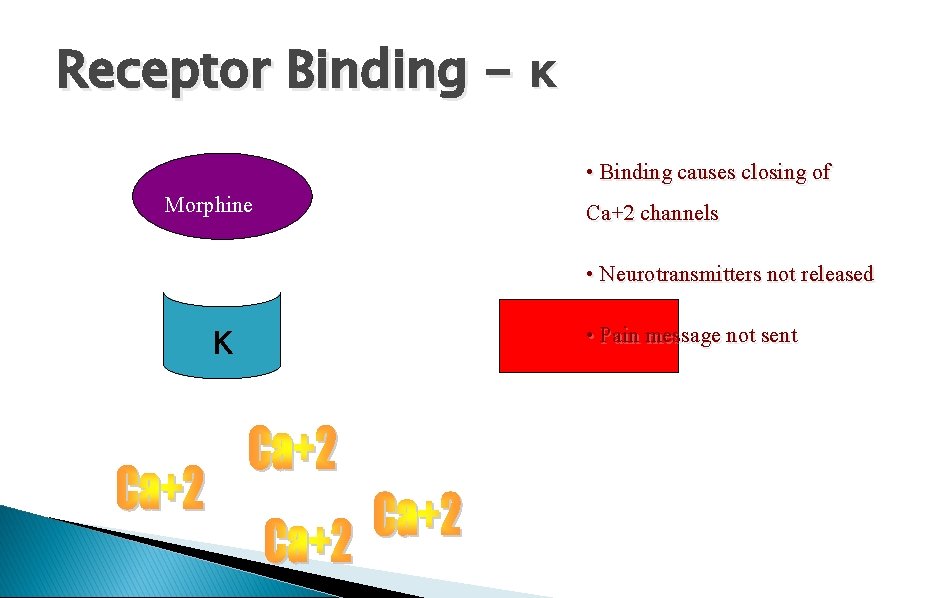
Receptor Binding - κ Morphine • Binding causes closing of Ca+2 channels • Neurotransmitters not released κ • Pain message not sent

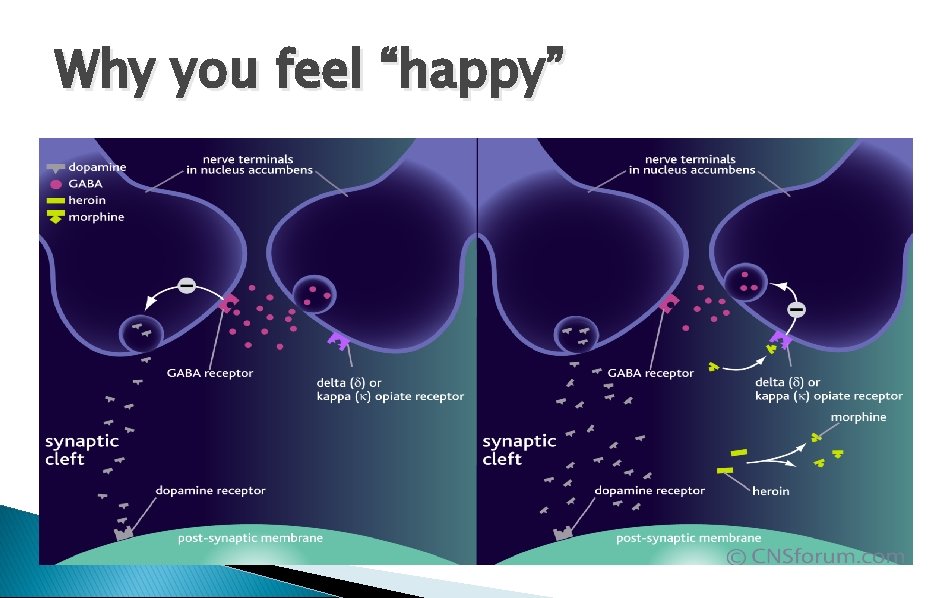
Why you feel “happy”

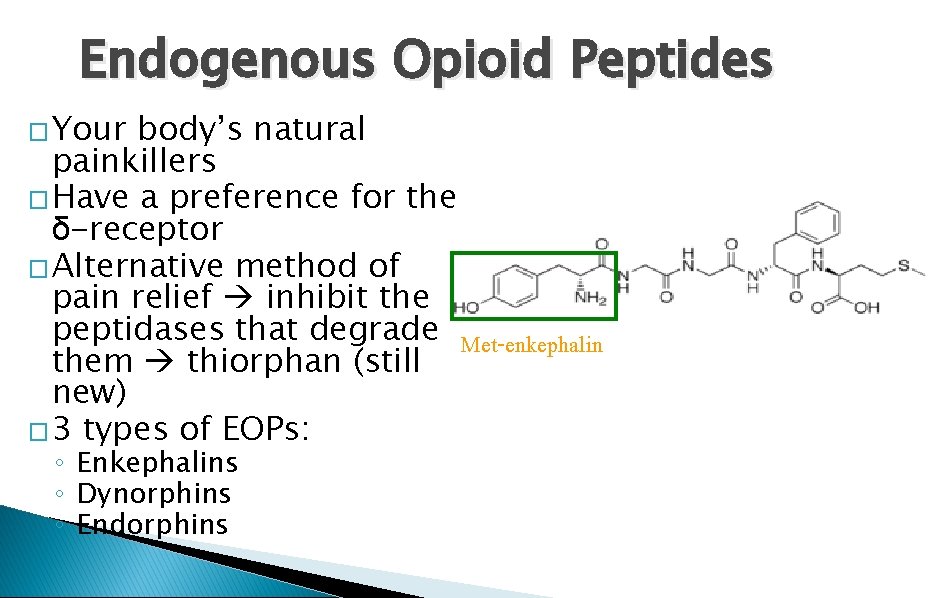
Endogenous Opioid Peptides � Your body’s natural painkillers � Have a preference for the δ-receptor � Alternative method of pain relief inhibit the peptidases that degrade Met-enkephalin them thiorphan (still new) � 3 types of EOPs: ◦ Enkephalins ◦ Dynorphins ◦ Endorphins

Partial Kappa Agonist

Receptor binding sites 1)Essential binding sites: center phenolic-OH, Ar, ionized N 2 (Agonist binding sites (Hydrophobic( 3 (Antagonist binding sites (Hydrophobic(

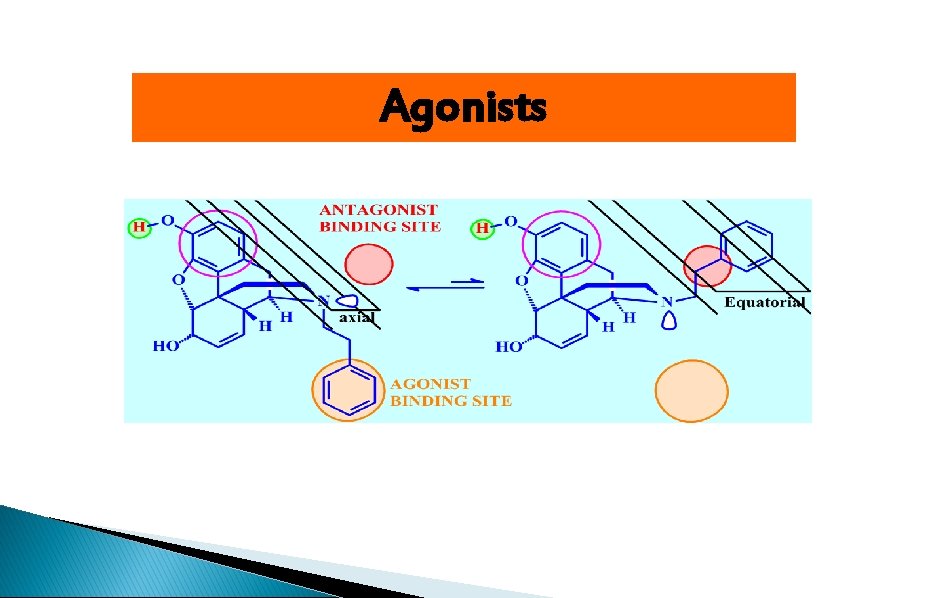
Agonists

5. 2 Partial agonist

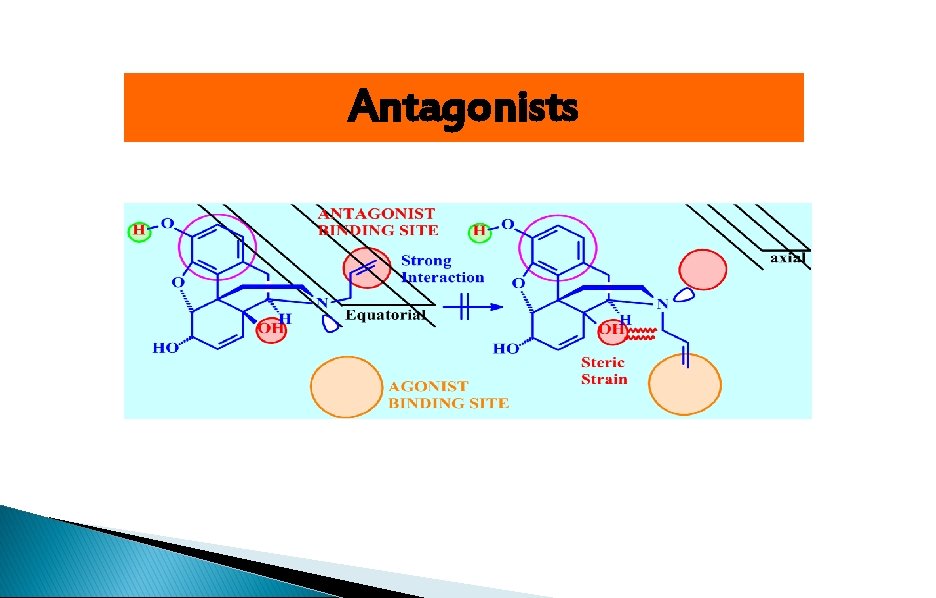
Antagonists

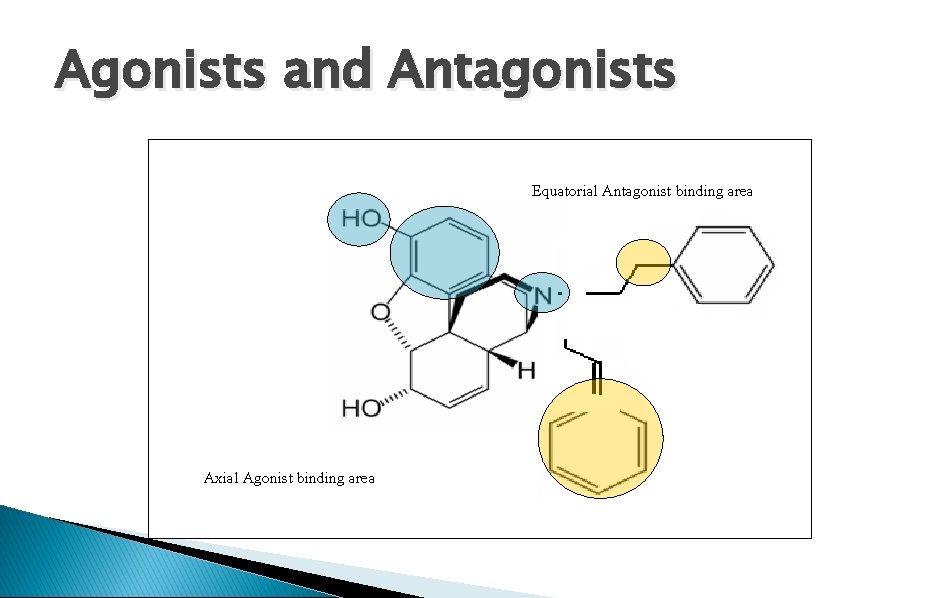
Agonists and Antagonists Equatorial Antagonist binding area Axial Agonist binding area

1. morphine 2. peripheral modification: codeine, 6 - acetylmorphine, heroin, hydromorphone, hydrocodone, oxymorphone, oxycodone 3. drug extention: naloxone, naltrexone, nalmefene, nalorphine

4. simplification Morphinans: 1. ring D is not essential 2. more active, loger acting but more SE than morphine: Levorphanol: orally active 3. Morphinans and Morphine: reacting with the same receptors in the same way. Benzomorphans: 1. ring C, D are not essential 2. analgesia and addiction are not necessarily coexistent 3. reasonable analgesic activity, less addictive liability, less tolerance simpler to synthesize 4. phenazocene, pentazocine 5. hallucinogenic effect

4 -Phenylpiperidine 1. ring C, D, E are not essential 2. retain SE: addition, respiratory depression 3. faster acting, shorter duration of action 4. interact with the analgesic receptors in a different manner to morphine 5. meperidine (pethidine), fentanyl, carfentanil, lofentanil, sufentanyl, alfentanil, remifentanil Diphenylpropylamine: methadone 1. ring B, C, D, E are not essential 2. comparable in activity to morhine 3. orally active 4. retains morphines-like SE: - less severe sedation, euphoria, withdrawal, emetic, constipation 5. a substitute for morphine or heroin

5. rigidification etorphine, buprenorphine

SIDE NOTE: � Other factors important to receptor binding: ◦ Stereochemistry �Enantiomers of many of the analogues were tested for analgesic activity. Overall, they didn’t have any. ◦ Rigidification �Used to maintain active formation and eliminate alternative conformations �Increases selectivity for receptors

Kappa agonist: Selective K-agonist (Not Specific(

The Future � Find an agonist that solely binds to the κreceptor � Explore the μ-receptor subtypes further to see if any of them don’t cause harmful side effects � Peripheral opiate receptors – avoid BBB obstacle � Block postsynaptic receptors involved in the transmission of a pain signal � GABA � Agonists for the cannabinoid receptor

2. Selective Mu 1 agonist 3. Peripheral opiate receptor agonist

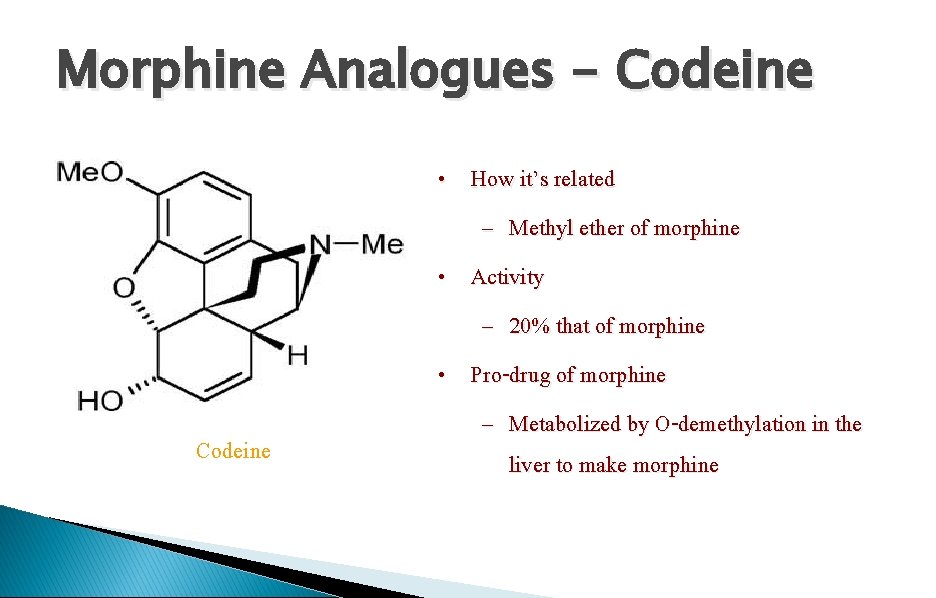
Morphine Analogues - Codeine • How it’s related – Methyl ether of morphine • Activity – 20% that of morphine • Pro-drug of morphine – Metabolized by O-demethylation in the liver to make morphine

Morphine Analogues - Codeine � Treats: ◦ Moderate pain ◦ Coughs ◦ diarrhea � Marketed as: ◦ Tylenol® with Codeine ◦ Hydrocodone ◦ Vicodin® (with Thebaine)

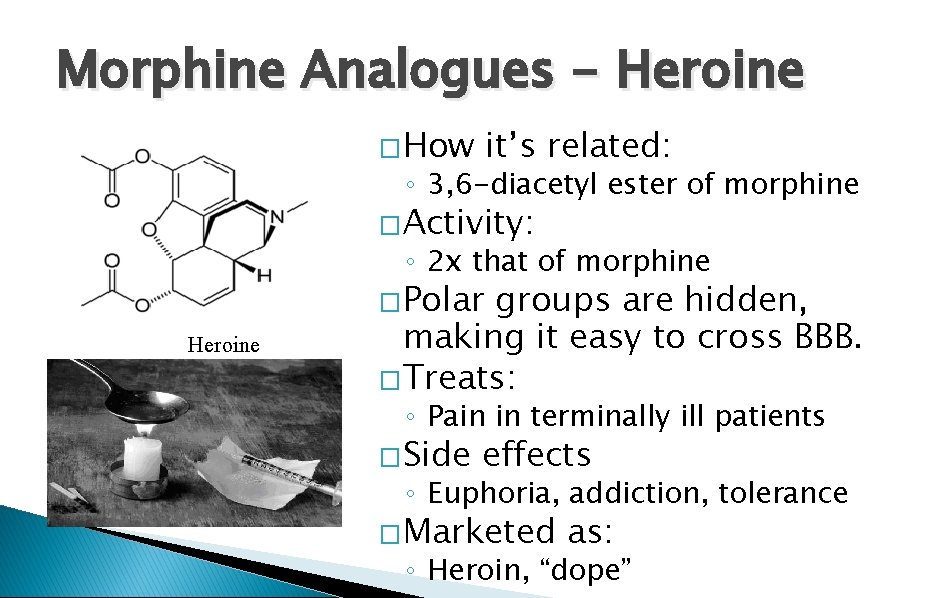
Morphine Analogues - Heroine � How it’s related: ◦ 3, 6 -diacetyl ester of morphine � Activity: ◦ 2 x that of morphine Heroine � Polar groups are hidden, making it easy to cross BBB. � Treats: ◦ Pain in terminally ill patients � Side effects ◦ Euphoria, addiction, tolerance � Marketed as: ◦ Heroin, “dope”

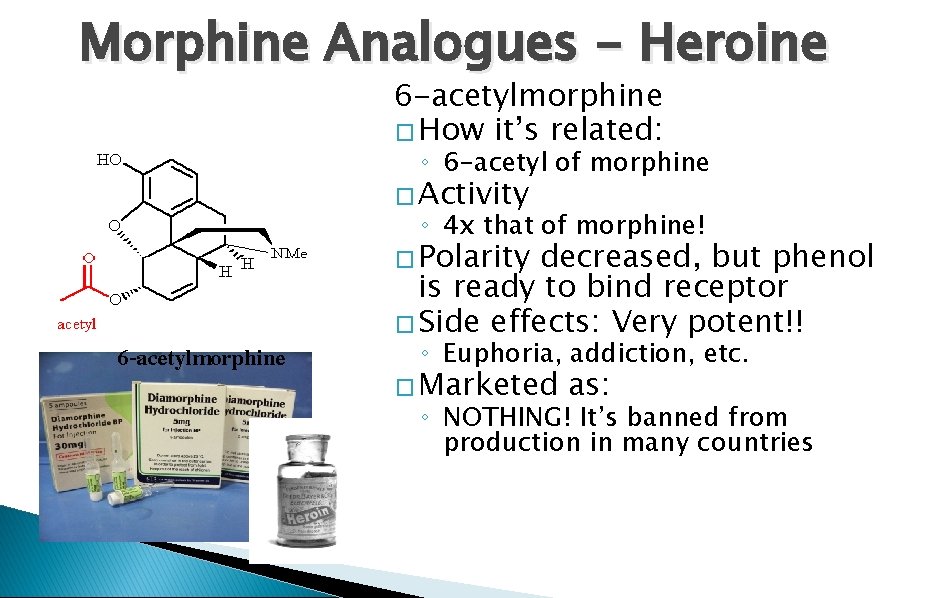
Morphine Analogues - Heroine 6 -acetylmorphine � How it’s related: ◦ 6 -acetyl of morphine � Activity ◦ 4 x that of morphine! � Polarity 6 -acetylmorphine decreased, but phenol is ready to bind receptor � Side effects: Very potent!! ◦ Euphoria, addiction, etc. � Marketed as: ◦ NOTHING! It’s banned from production in many countries

http: //www. opioids. com Patrick, G. L. 1995. An introduction to medicinal chemistry. Oxford: Oxford University Press. Wolff, M. E. 1995. Burger’s medicinal chemistry and drug discovery. vol. 1. 5 th ed. New York: John Wiley & Sons.
 Morpheus greek god
Morpheus greek god Who is neptune named after
Who is neptune named after Venus is named after
Venus is named after Mercury planet
Mercury planet After me after me after me
After me after me after me If any man wants to come after me
If any man wants to come after me Https://www.retourabsence.bscc.fr
Https://www.retourabsence.bscc.fr Relative configuration
Relative configuration Equianalgesie morphine
Equianalgesie morphine History of morphine
History of morphine Obat dari morphine tts
Obat dari morphine tts Morpheus bms
Morpheus bms Morpheus coca cola
Morpheus coca cola Morpheus foundation
Morpheus foundation Teddy bear museum naples
Teddy bear museum naples Juno awards date
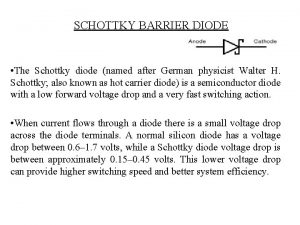
Juno awards date Why schottky diode is called hot carrier diode
Why schottky diode is called hot carrier diode Rogerian argument outline
Rogerian argument outline What was america named after
What was america named after Jamestown
Jamestown Headright system meaning
Headright system meaning Harvard demographics
Harvard demographics Cartesian plane named after
Cartesian plane named after Is the victorian era named after queen victoria
Is the victorian era named after queen victoria What is ares realm
What is ares realm Why is athens named after athena
Why is athens named after athena Pareto graph definition
Pareto graph definition Who is neptune named after
Who is neptune named after Where was the first fbla chapter located
Where was the first fbla chapter located Boolean expressions was named after him.
Boolean expressions was named after him. Determines who wins most games candy bar
Determines who wins most games candy bar Ethos, pathos and logos
Ethos, pathos and logos What is the greek miracle in greek mythology
What is the greek miracle in greek mythology Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Số nguyên tố là số gì
Số nguyên tố là số gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Questioning faith after death
Questioning faith after death Zeus temper affected
Zeus temper affected Greek god orion
Greek god orion Greek word for kingdom of god
Greek word for kingdom of god What is zeus weakness
What is zeus weakness