More complex stoichiometry problem done 2 ways MW

More complex stoichiometry problem done 2 ways MW 44 32 44 18 C 3 H 8+ 5 O 2 3 CO 2+ 4 H 2 O g/mol 22 grams of C 3 H 8 burned with O 2 makes how many grams of H 2 O ? Method 1: factor label 22 g C 3 H 8 x 1 mole C 3 H 8 x 4 mol H 2 O 44 g C 3 H 8 1) Given 1 mole C 3 H 8 x 18 g H 2 O 1 mol H 2 O = 36? ? g H 2 O 2) Want 3)Find ratios that lead to desired units from reaction and MW …which ? ? ? . . . do on board
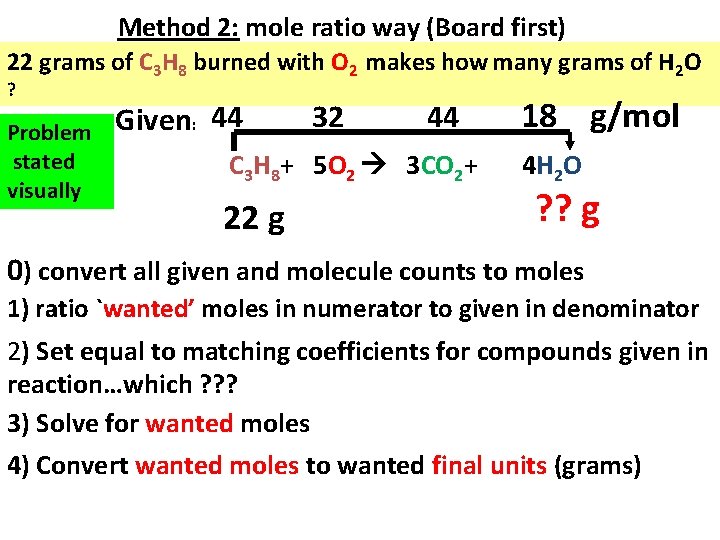
Method 2: mole ratio way (Board first) 22 grams of C 3 H 8 burned with O 2 makes how many grams of H 2 O ? Problem stated visually Given: 44 32 44 C 3 H 8+ 5 O 2 3 CO 2+ 22 g 18 g/mol 4 H 2 O ? ? g 0) convert all given and molecule counts to moles 1) ratio `wanted’ moles in numerator to given in denominator 2) Set equal to matching coefficients for compounds given in reaction…which ? ? ? 3) Solve for wanted moles 4) Convert wanted moles to wanted final units (grams)
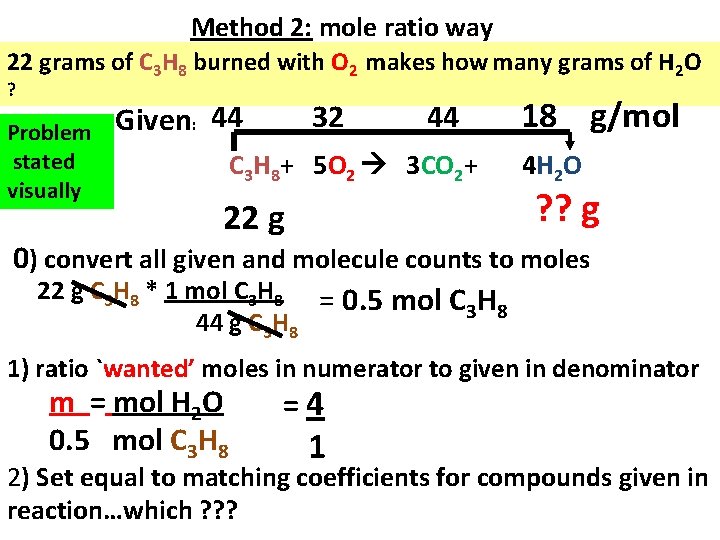
Method 2: mole ratio way 22 grams of C 3 H 8 burned with O 2 makes how many grams of H 2 O ? Problem stated visually Given: 44 32 44 C 3 H 8+ 5 O 2 3 CO 2+ 22 g 18 g/mol 4 H 2 O ? ? g 0) convert all given and molecule counts to moles 22 g C 3 H 8 * 1 mol C 3 H 8 = 0. 5 mol C H 3 8 44 g C 3 H 8 1) ratio `wanted’ moles in numerator to given in denominator m = mol H 2 O 0. 5 mol C 3 H 8 =4 1 2) Set equal to matching coefficients for compounds given in reaction…which ? ? ?
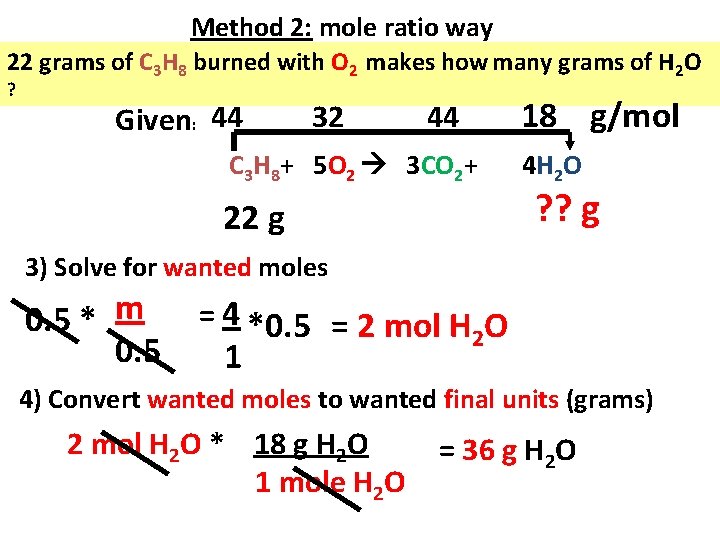
Method 2: mole ratio way 22 grams of C 3 H 8 burned with O 2 makes how many grams of H 2 O ? Given: 44 32 44 C 3 H 8+ 5 O 2 3 CO 2+ 22 g 18 g/mol 4 H 2 O ? ? g 3) Solve for wanted moles 0. 5 * m 0. 5 = 4 *0. 5 = 2 mol H O 2 1 4) Convert wanted moles to wanted final units (grams) 2 mol H 2 O * 18 g H 2 O 1 mole H 2 O = 36 g H 2 O
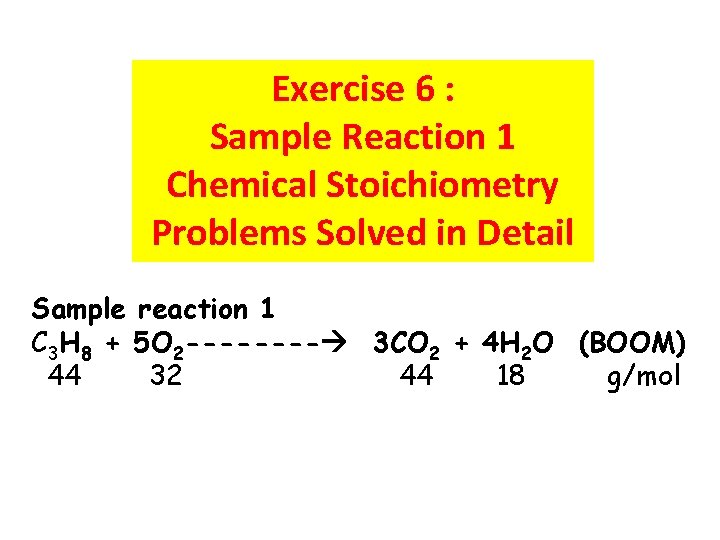
Exercise 6 : Sample Reaction 1 Chemical Stoichiometry Problems Solved in Detail Sample reaction 1 C 3 H 8 + 5 O 2 ---- 3 CO 2 + 4 H 2 O (BOOM) 44 32 44 18 g/mol
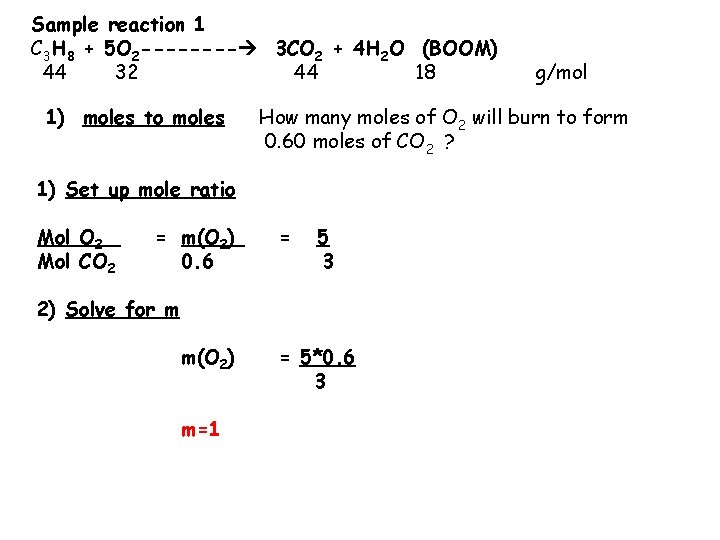
Sample reaction 1 C 3 H 8 + 5 O 2 ---- 3 CO 2 + 4 H 2 O (BOOM) 44 32 44 18 1) moles to moles How many moles of O 2 will burn to form 0. 60 moles of CO 2 ? 1) Set up mole ratio Mol O 2 Mol CO 2 = m(O 2) 0. 6 = 5 3 2) Solve for m m(O 2) m=1 g/mol = 5*0. 6 3
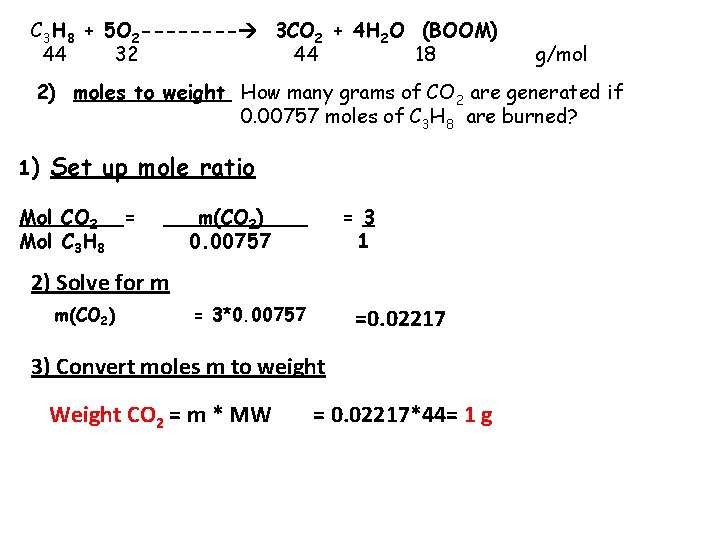
C 3 H 8 + 5 O 2 ---- 3 CO 2 + 4 H 2 O (BOOM) 44 32 44 18 g/mol 2) moles to weight How many grams of CO 2 are generated if 0. 00757 moles of C 3 H 8 are burned? 1) Set up mole ratio Mol CO 2 = Mol C 3 H 8 m(CO 2)_ 0. 00757 = 3 1 2) Solve for m m(CO 2) = 3*0. 00757 =0. 02217 3) Convert moles m to weight Weight CO 2 = m * MW = 0. 02217*44= 1 g
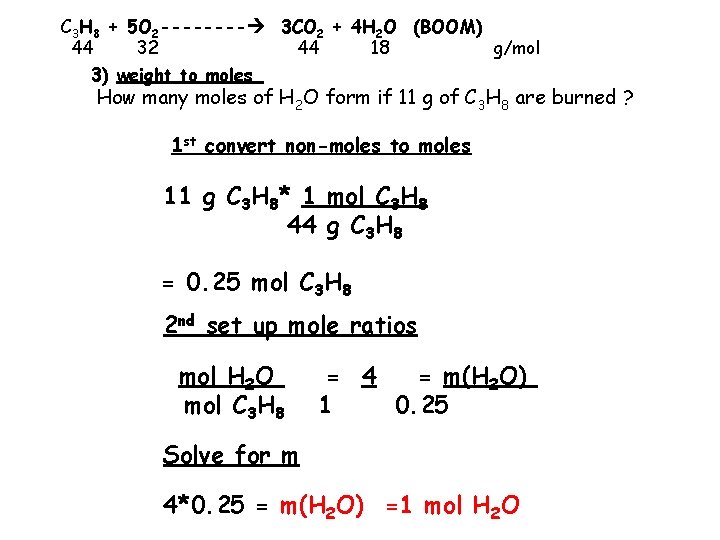
C 3 H 8 + 5 O 2 ---- 3 CO 2 + 4 H 2 O (BOOM) 44 32 44 18 g/mol 3) weight to moles How many moles of H 2 O form if 11 g of C 3 H 8 are burned ? 1 st convert non-moles to moles 11 g C 3 H 8* 1 mol C 3 H 8 44 g C 3 H 8 = 0. 25 mol C 3 H 8 2 nd set up mole ratios mol H 2 O mol C 3 H 8 = 4 = m(H 2 O) 1 0. 25 Solve for m 4*0. 25 = m(H 2 O) =1 mol H 2 O
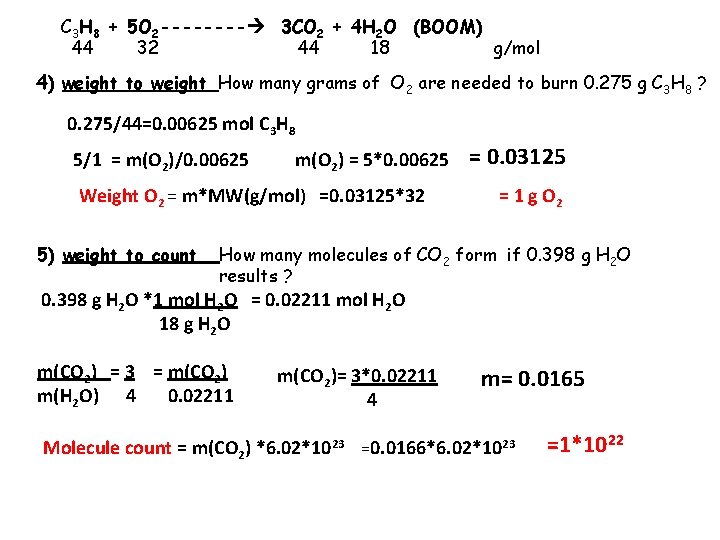
C 3 H 8 + 5 O 2 ---- 3 CO 2 + 4 H 2 O (BOOM) 44 32 44 18 g/mol 4) weight to weight How many grams of O 2 are needed to burn 0. 275 g C 3 H 8 ? 0. 275/44=0. 00625 mol C 3 H 8 5/1 = m(O 2)/0. 00625 m(O 2) = 5*0. 00625 Weight O 2 = m*MW(g/mol) =0. 03125*32 5) weight to count = 0. 03125 = 1 g O 2 How many molecules of CO 2 form if 0. 398 g H 2 O results ? 0. 398 g H 2 O *1 mol H 2 O = 0. 02211 mol H 2 O 18 g H 2 O m(CO 2) = 3 = m(CO 2) m(H 2 O) 4 0. 02211 m(CO 2)= 3*0. 02211 4 m= 0. 0165 Molecule count = m(CO 2) *6. 02*1023 =0. 0166*6. 02*1023 =1*1022
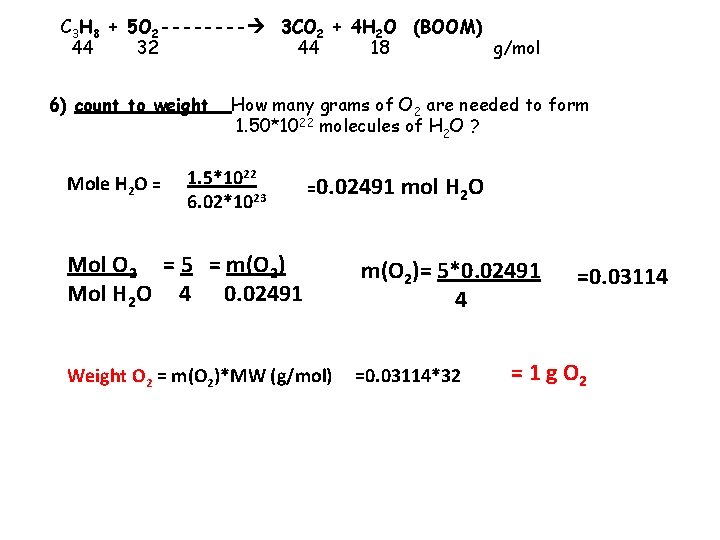
C 3 H 8 + 5 O 2 ---- 3 CO 2 + 4 H 2 O (BOOM) 44 32 44 18 g/mol 6) count to weight Mole H 2 O = How many grams of O 2 are needed to form 1. 50*1022 molecules of H 2 O ? 1. 5*1022 6. 02*1023 =0. 02491 mol H 2 O Mol O 2 = 5 = m(O 2) Mol H 2 O 4 0. 02491 m(O 2)= 5*0. 02491 4 Weight O 2 = m(O 2)*MW (g/mol) =0. 03114*32 =0. 03114 = 1 g O 2
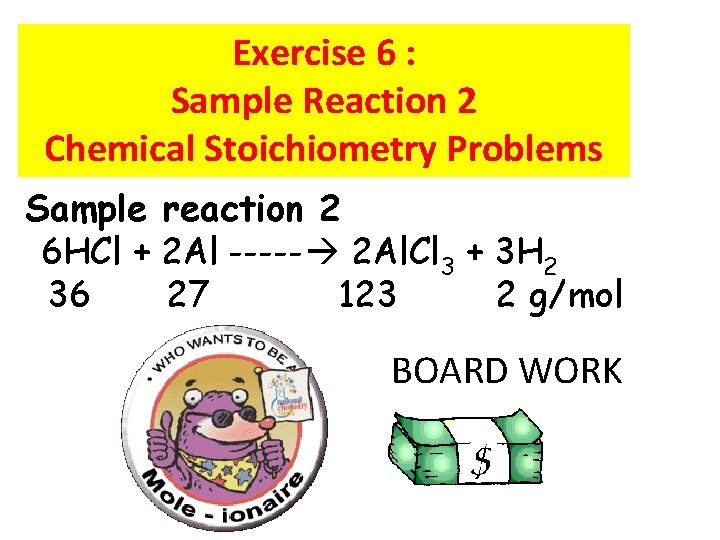
Exercise 6 : Sample Reaction 2 Chemical Stoichiometry Problems Sample reaction 2 6 HCl + 2 Al ----- 2 Al. Cl 3 + 3 H 2 36 27 123 2 g/mol BOARD WORK
- Slides: 11