More Atomic History Neon Neon Anode Cathode Anode









- Slides: 27

More Atomic History!

Neon

Neon

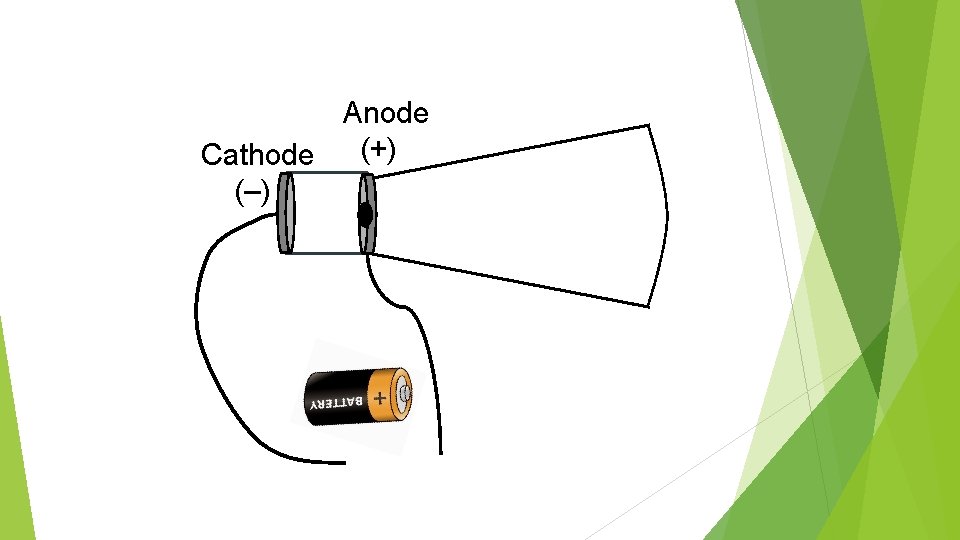
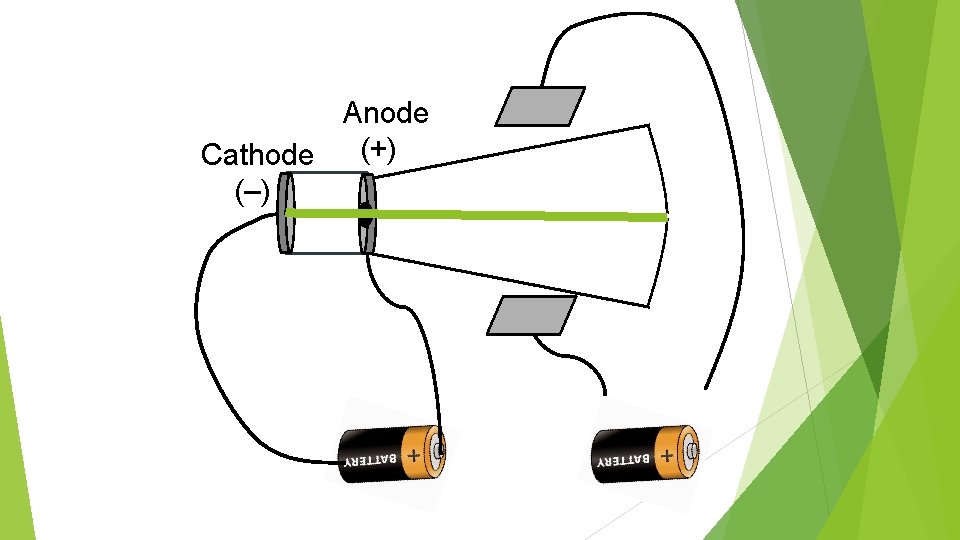
Anode (+) Cathode (–)

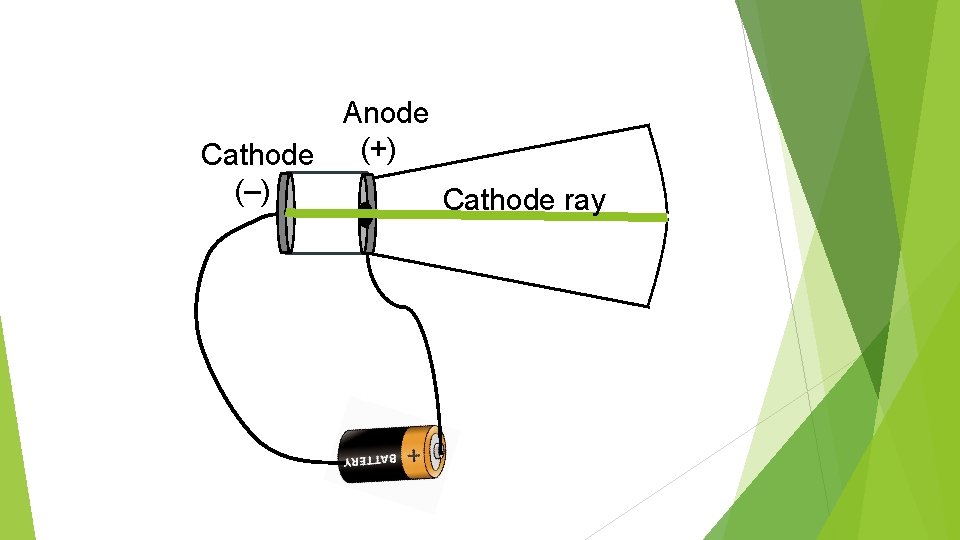
Anode (+) Cathode (–) Cathode ray

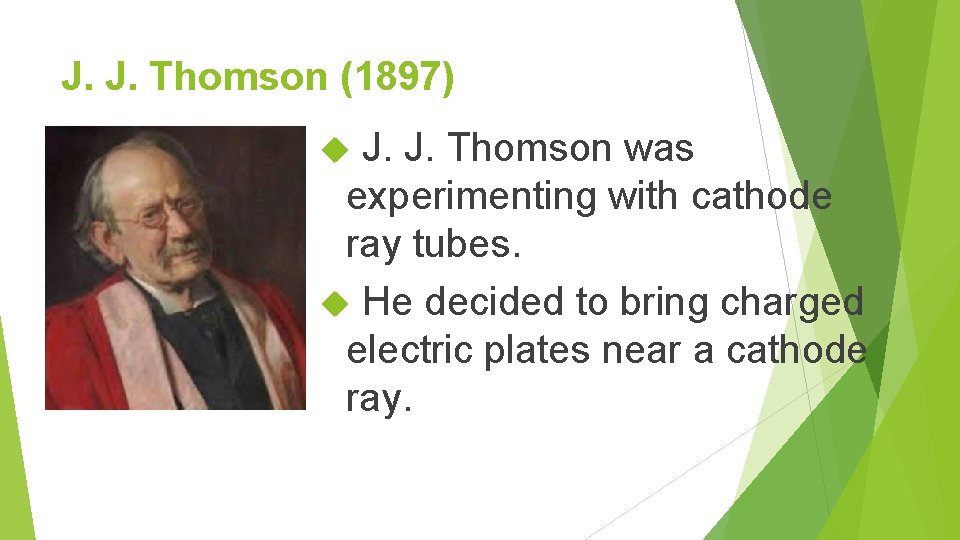
J. J. Thomson (1897) J. J. Thomson was experimenting with cathode ray tubes. He decided to bring charged electric plates near a cathode ray.

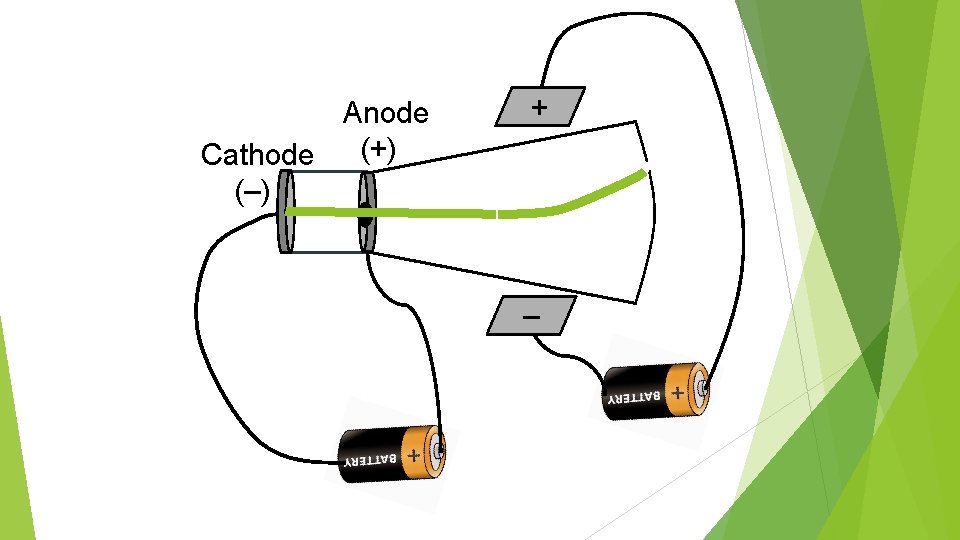
Anode (+) Cathode (–)

Anode (+) Cathode (–) + –

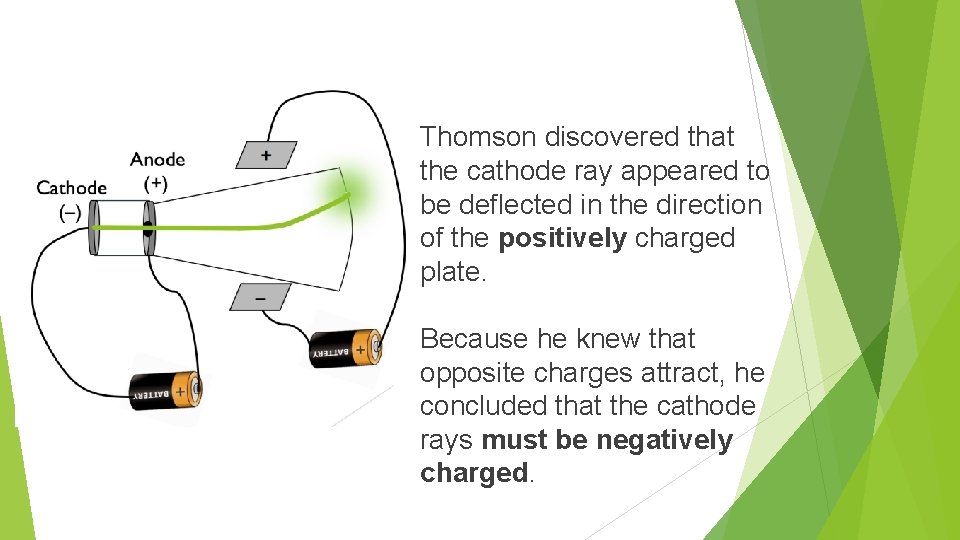
Thomson discovered that the cathode ray appeared to be deflected in the direction of the positively charged plate. Because he knew that opposite charges attract, he concluded that the cathode rays must be negatively charged.

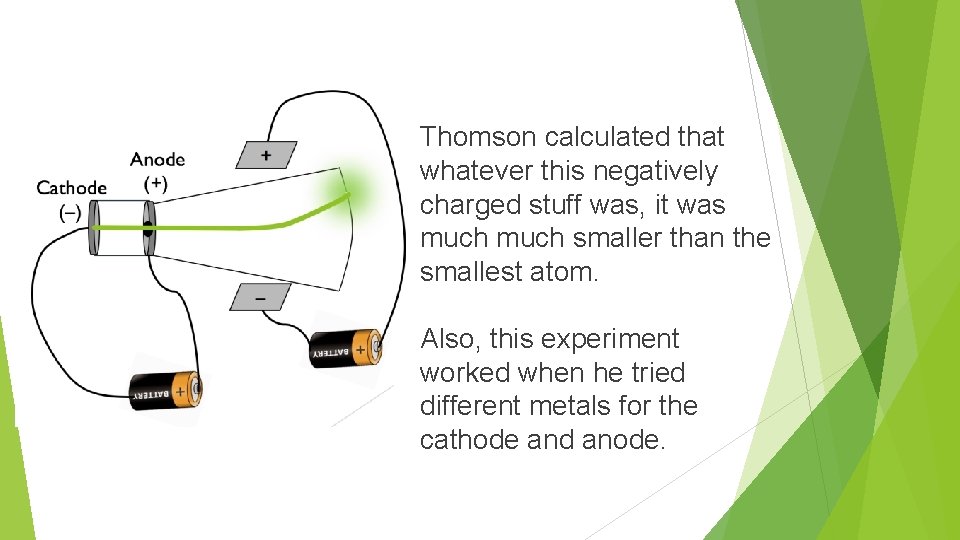
Thomson calculated that whatever this negatively charged stuff was, it was much smaller than the smallest atom. Also, this experiment worked when he tried different metals for the cathode and anode.

Dalton Timbit Models

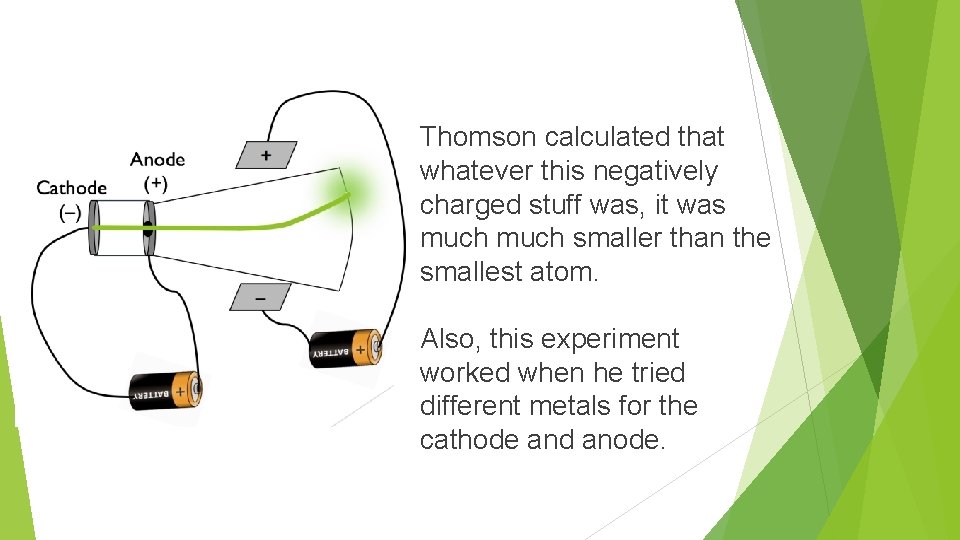
Thomson calculated that whatever this negatively charged stuff was, it was much smaller than the smallest atom. Also, this experiment worked when he tried different metals for the cathode and anode.

Thomson’s Conclusion Atoms contain negatively charged particles. These are called electrons.

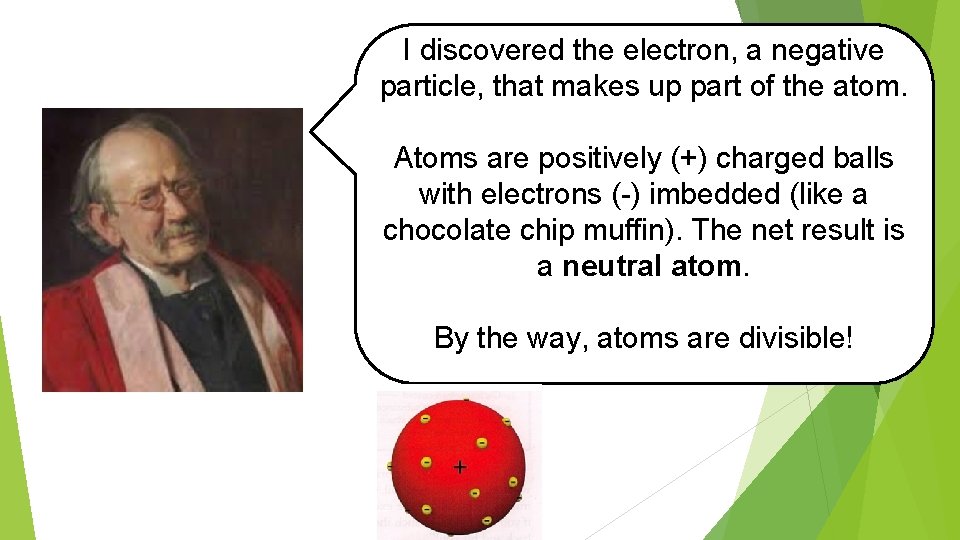
I discovered the electron, a negative particle, that makes up part of the atom. Atoms are positively (+) charged balls with electrons (-) imbedded (like a chocolate chip muffin). The net result is a neutral atom. By the way, atoms are divisible! !

Thomson’s model was nicknamed the “plum pudding model”.

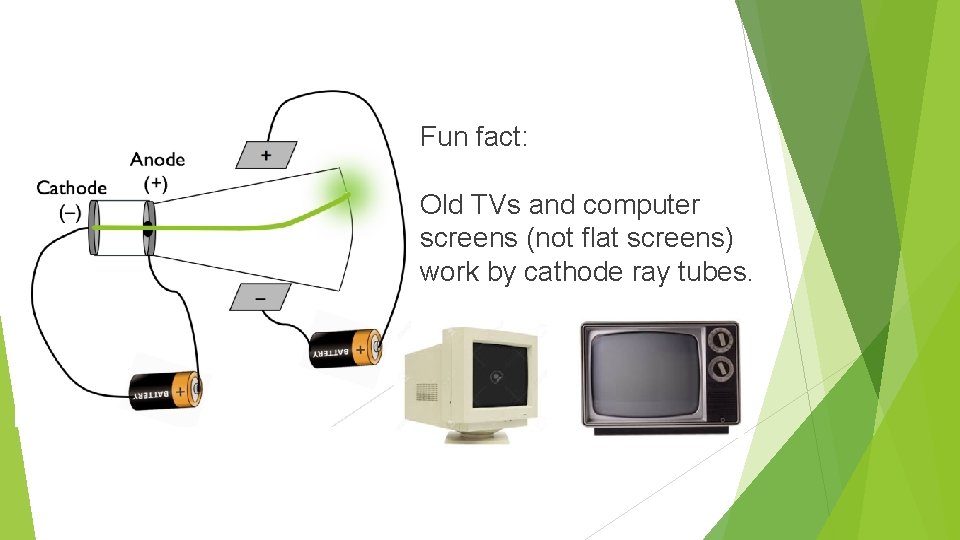
Fun fact: Old TVs and computer screens (not flat screens) work by cathode ray tubes.

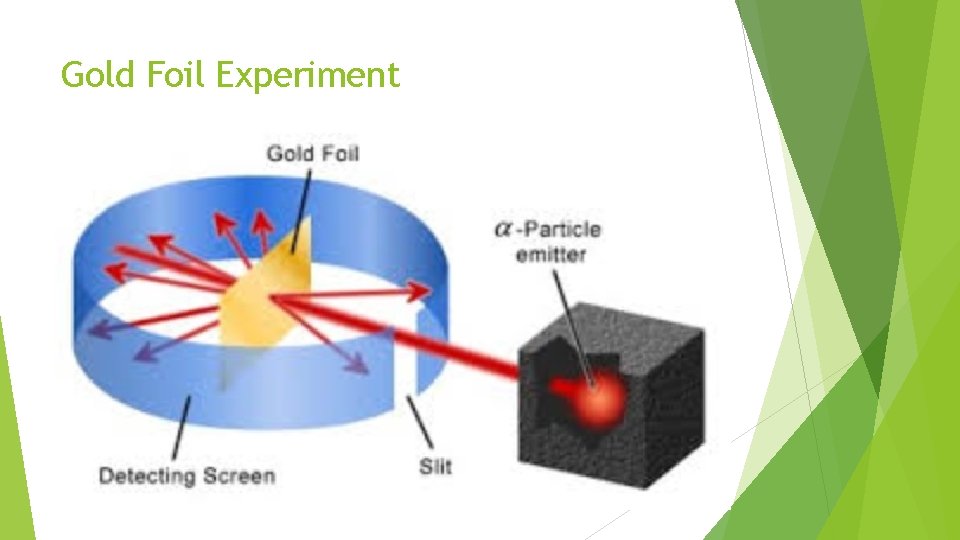
Ernest Rutherford Radioactive substances can give off alpha (+), beta (-) and gamma (neutral) radiation. I bombarded gold foil with alpha (+) particles. Ernest Rutherford, 1911 !

Ernest Rutherford Radioactive substances can give off alpha (+), beta (-) and gamma (neutral) radiation. I bombarded gold foil with alpha (+) particles. Ernest Rutherford, 1911 !


Gold Foil Experiment

2 conclusions Atoms An are mostly empty space. atom contains a very dense and small nucleus. The nucleus is positively charged.

2 conclusions Atoms An are mostly empty space. atom contains a very dense and small nucleus. The nucleus is positively charged.


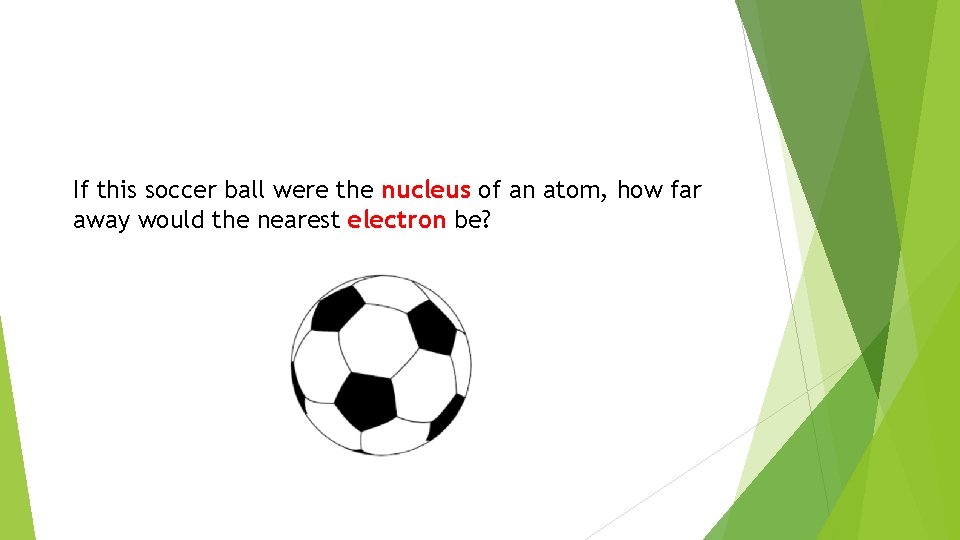
If this soccer ball were the nucleus of an atom, how far away would the nearest electron be?



What can we conclude from this? Atoms are mostly empty space.