Monoterapia o combinacin Delvys Rodrguez Abreu MD Medical

¿Monoterapia o combinación? Delvys Rodríguez Abreu, MD Medical Oncology Unit Hospital Universitario Insular de Gran Canaria, Spain

Disclosure Information • Consultant or Advisory Role: BMS, MSD, GENENTECH/ROCHE, ASTRA ZENECA, BOEHRINGER INGELHEIM, NOVARTIS, Pfizer, Lilly. • Lectures: BMS, MSD, GENENTECH/ROCHE, ASTRA ZENECA, BOEHRINGER INGELHEIM, Lilly, Pfizer. • Grant support for studies: BMS • Disclaimer: Con el fin de dar una vision balanceada, completa y actualizada de la situación del tratamiento del cancer de pulmón metastásico, se incluyen en algunas partes de esta presentación datos de ensayos clínicos de diferentes moléculas, en proceso de aprobación por la EMA.
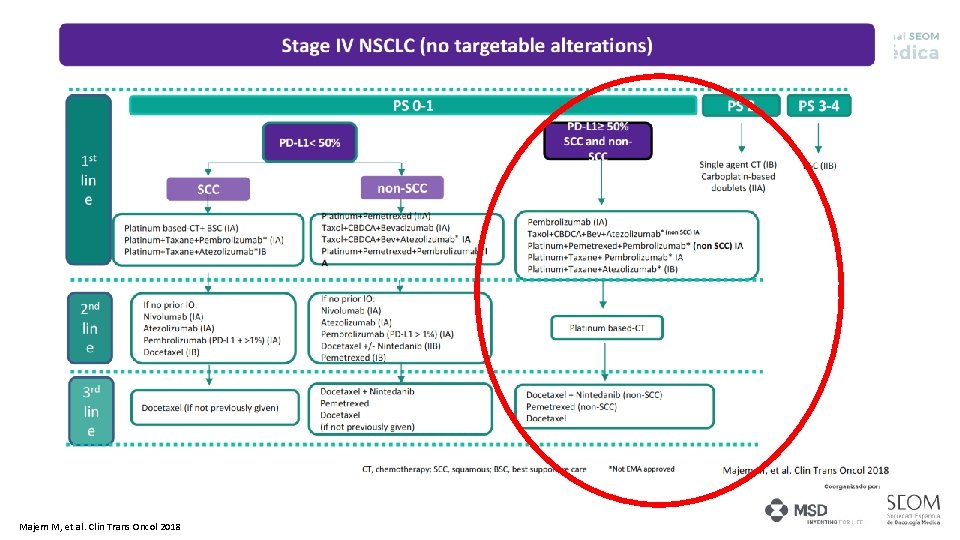
Majem M, et al. Clin Trans Oncol 2018
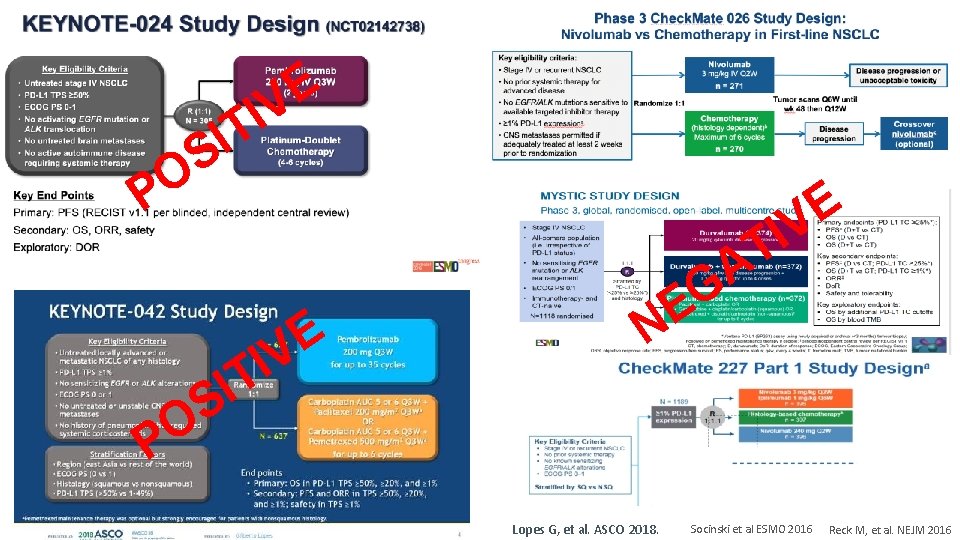
E V I T I S O P E IV T A G E N Lopes G, et al. ASCO 2018. Socinski et al ESMO 2016 Reck M, et al. NEJM 2016
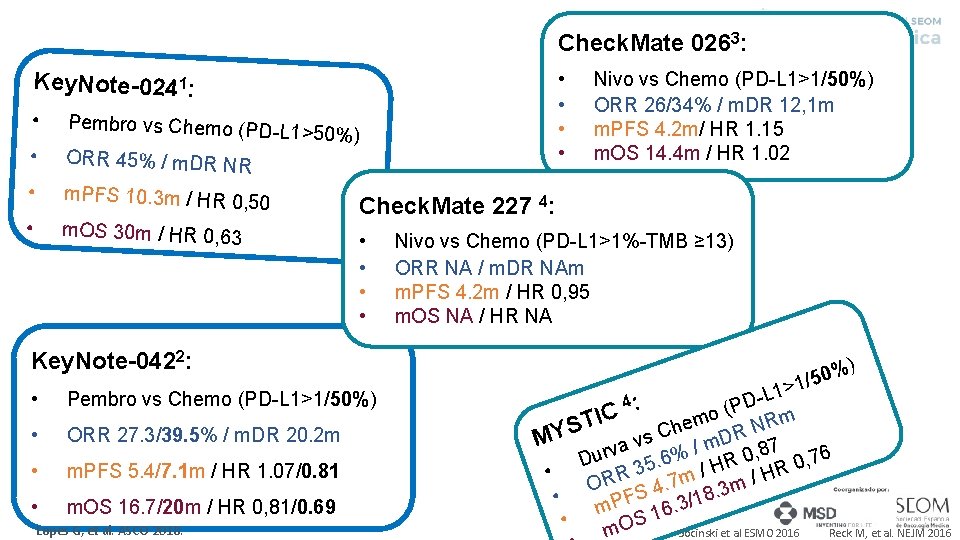
Check. Mate 0263: • • Key. Note-0241: • Pembro vs Chemo (PD -L • ORR 45% / m. DR NR • m. PFS 10. 3 m / HR 0, 50 • m. OS 30 m / HR 0, 63 1>50%) Nivo vs Chemo (PD-L 1>1/50%) ORR 26/34% / m. DR 12, 1 m m. PFS 4. 2 m/ HR 1. 15 m. OS 14. 4 m / HR 1. 02 Check. Mate 227 4: • • Nivo vs Chemo (PD-L 1>1%-TMB ≥ 13) ORR NA / m. DR NAm m. PFS 4. 2 m / HR 0, 95 m. OS NA / HR NA Key. Note-0422: • Pembro vs Chemo (PD-L 1>1/50%) • ORR 27. 3/39. 5% / m. DR 20. 2 m • m. PFS 5. 4/7. 1 m / HR 1. 07/0. 81 • m. OS 16. 7/20 m / HR 0, 81/0. 69 Lopes G, et al. ASCO 2018. 0% 5 / 1 > ) L 1 D (P o C I m Rm T e N h S C s DR 7 MY v m a / v 8 Dur 35. 6% HR 0, 76 / • R m /H R 7. O m 4 S 8. 3 • F 1 / P 3 m 16. S • Reck M, et al. NEJM 2016 Socinski et al ESMO 2016 m. O 4:
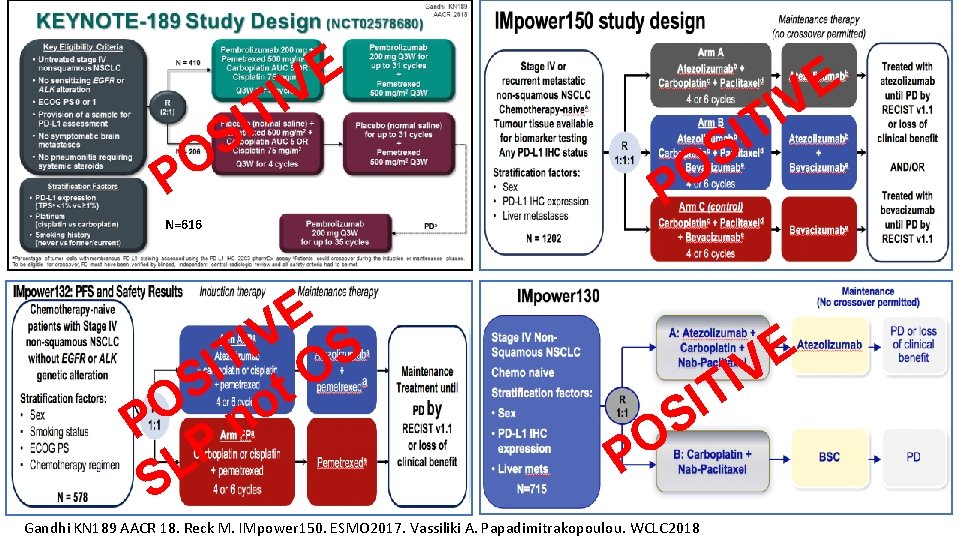
E V I T I S O P N=616 E V S I T O I S t O o n P P L S E V I T I S O P Gandhi KN 189 AACR 18. Reck M. IMpower 150. ESMO 2017. Vassiliki A. Papadimitrakopoulou. WCLC 2018
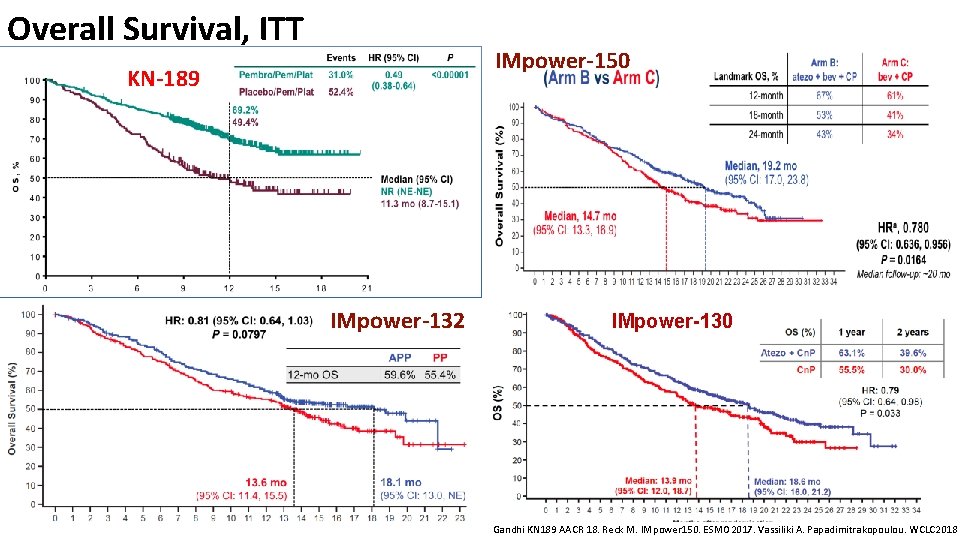
Overall Survival, ITT IMpower-150 KN-189 IMpower-132 IMpower-130 Gandhi KN 189 AACR 18. Reck M. IMpower 150. ESMO 2017. Vassiliki A. Papadimitrakopoulou. WCLC 2018
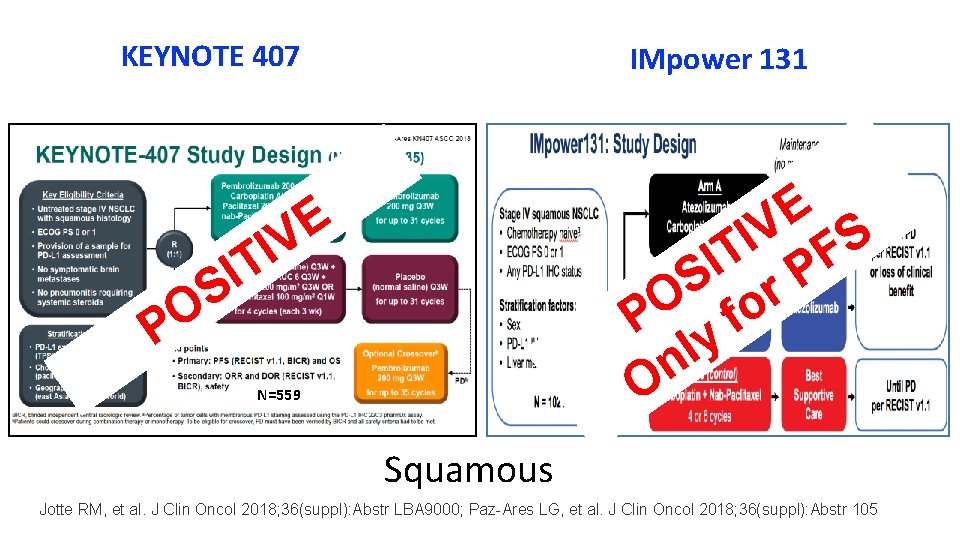
KEYNOTE 407 IMpower 131 E V S I T PF I S r O P y fo l n O E IV T I S O P N=559 Squamous Jotte RM, et al. J Clin Oncol 2018; 36(suppl): Abstr LBA 9000; Paz-Ares LG, et al. J Clin Oncol 2018; 36(suppl): Abstr 105
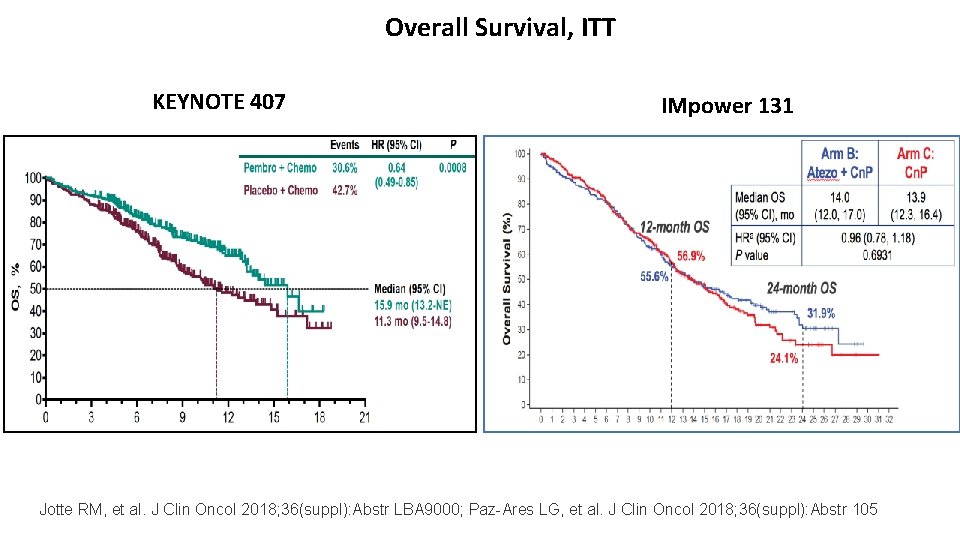
Overall Survival, ITT KEYNOTE 407 IMpower 131 Jotte RM, et al. J Clin Oncol 2018; 36(suppl): Abstr LBA 9000; Paz-Ares LG, et al. J Clin Oncol 2018; 36(suppl): Abstr 105

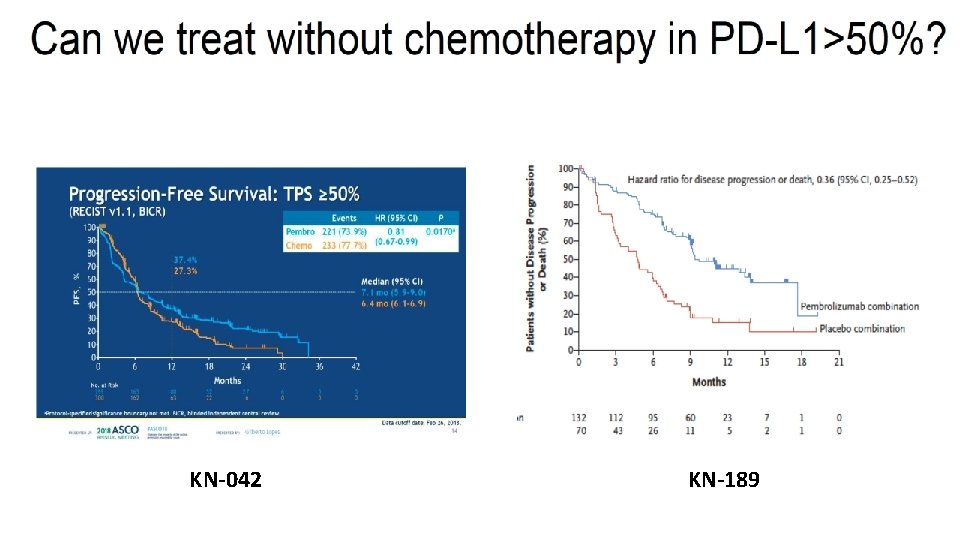
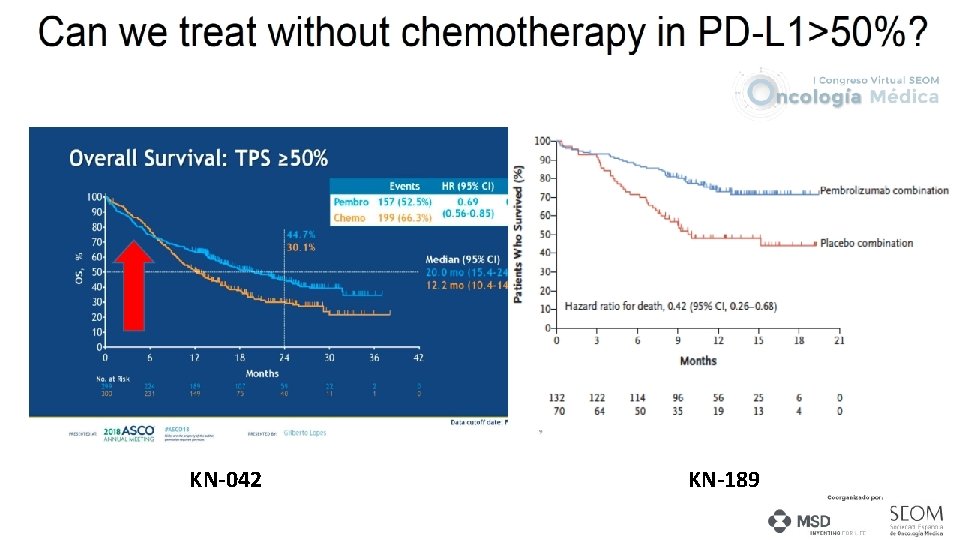
Can we avoid chemotherapy in the PD-L 1 >50% subgroup?
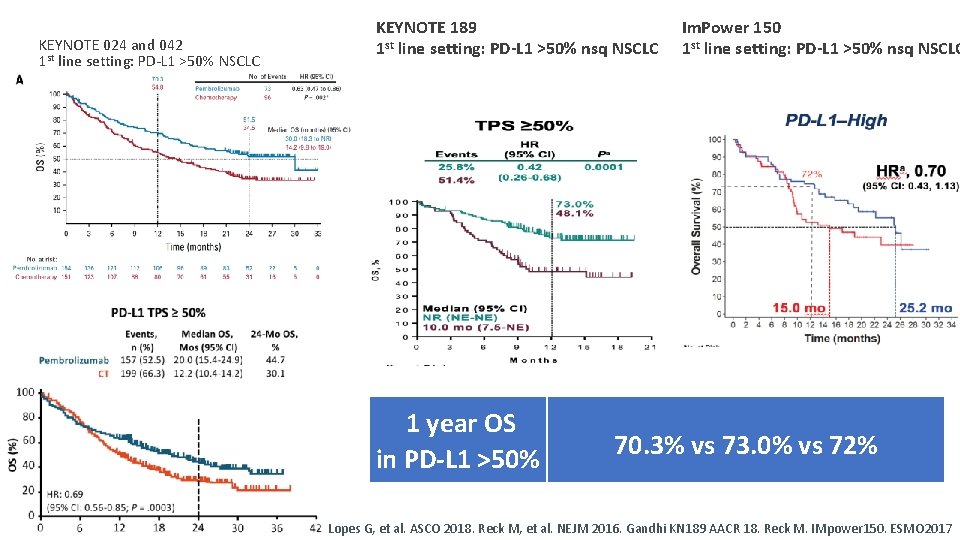
KEYNOTE 024 and 042 1 st line setting: PD-L 1 >50% NSCLC KEYNOTE 189 1 st line setting: PD-L 1 >50% nsq NSCLC 1 year OS in PD-L 1 >50% Reck M, et al. J Clin Oncol 2019 Im. Power 150 1 st line setting: PD-L 1 >50% nsq NSCLC 70. 3% vs 73. 0% vs 72% Lopes G, et al. ASCO 2018. Reck M, et al. NEJM 2016. Gandhi KN 189 AACR 18. Reck M. IMpower 150. ESMO 2017
KN-042 KN-189
KN-042 KN-189
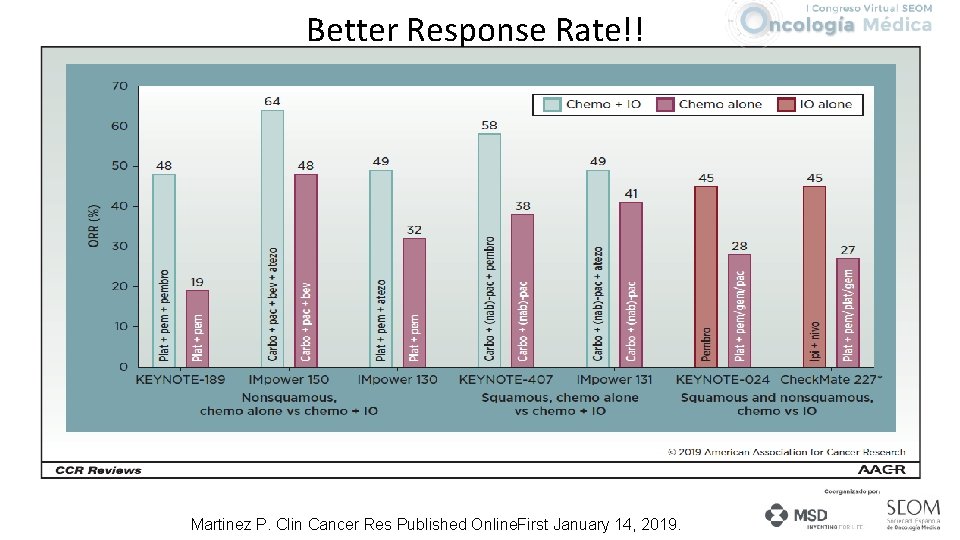
Better Response Rate!! Martinez P. Clin Cancer Res Published Online. First January 14, 2019.
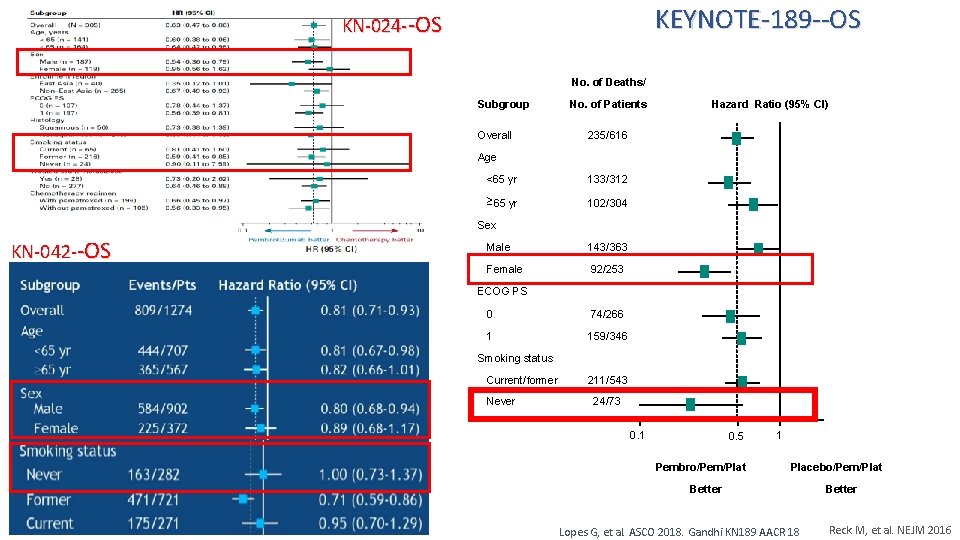
KEYNOTE-189 --OS KN-024 --OS No. of Deaths/ Subgroup Overall No. of Patients Hazard Ratio (95% CI) 235/616 Age <65 yr 133/312 ³ 65 yr 102/304 Sex KN-042 --OS Male 143/363 Female 92/253 ECOG PS 0 74/266 1 159/346 Smoking status Current/former Never 211/543 24/73 0. 1 0. 5 Pembro/Pem/Plat 1 Placebo/Pem/Plat Better Lopes G, et al. ASCO 2018. Gandhi KN 189 AACR 18 Better Reck M, et al. NEJM 2016
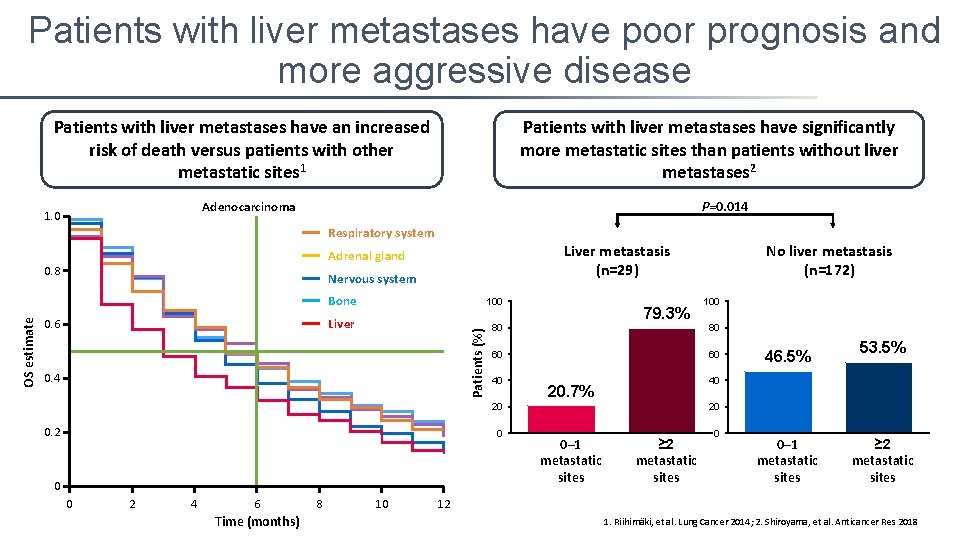
Patients with liver metastases have poor prognosis and more aggressive disease Patients with liver metastases have an increased risk of death versus patients with other metastatic sites 1 Patients with liver metastases have significantly more metastatic sites than patients without liver metastases 2 P=0. 014 Adenocarcinoma 1. 0 Respiratory system 0. 8 Nervous system 0. 6 Bone 100 Liver 80 Patients (%) OS estimate Liver metastasis (n=29) Adrenal gland 0. 4 79. 3% 60 40 0 2 4 6 Time (months) 8 10 100 80 60 46. 5% 53. 5% 40 20. 7% 20 0. 2 No liver metastasis (n=172) 20 0– 1 metastatic sites ≥ 2 metastatic sites 12 1. Riihimäki, et al. Lung Cancer 2014; 2. Shiroyama, et al. Anticancer Res 2018
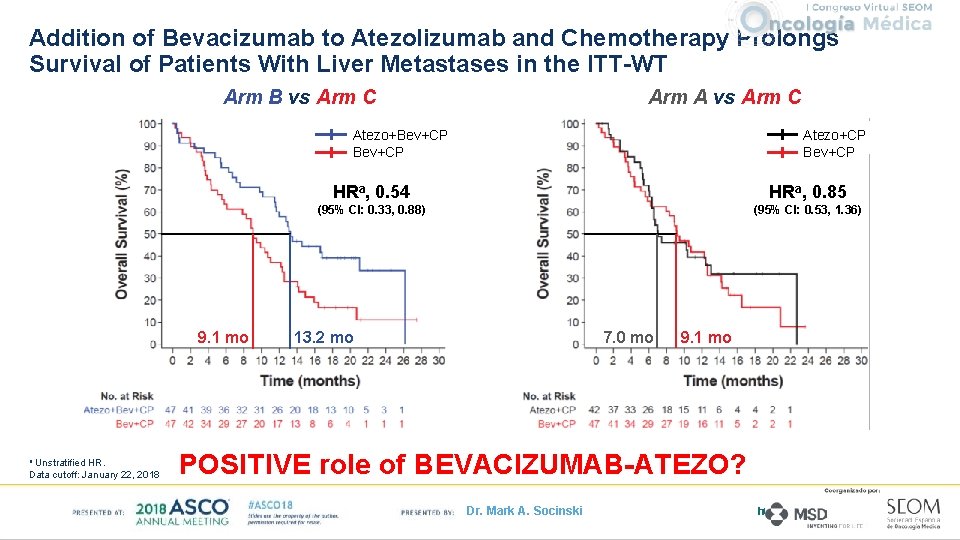
Addition of Bevacizumab to Atezolizumab and Chemotherapy Prolongs Survival of Patients With Liver Metastases in the ITT-WT Arm B vs Arm C Arm A vs Arm C Atezo+CP Bev+CP Atezo+Bev+CP 9. 1 mo Unstratified HR. Data cutoff: January 22, 2018 a HRa, 0. 54 HRa, 0. 85 (95% CI: 0. 33, 0. 88) (95% CI: 0. 53, 1. 36) 7. 0 mo 13. 2 mo 9. 1 mo POSITIVE role of BEVACIZUMAB-ATEZO? Dr. Mark A. Socinski http: //clicktoedit. URL. com 17
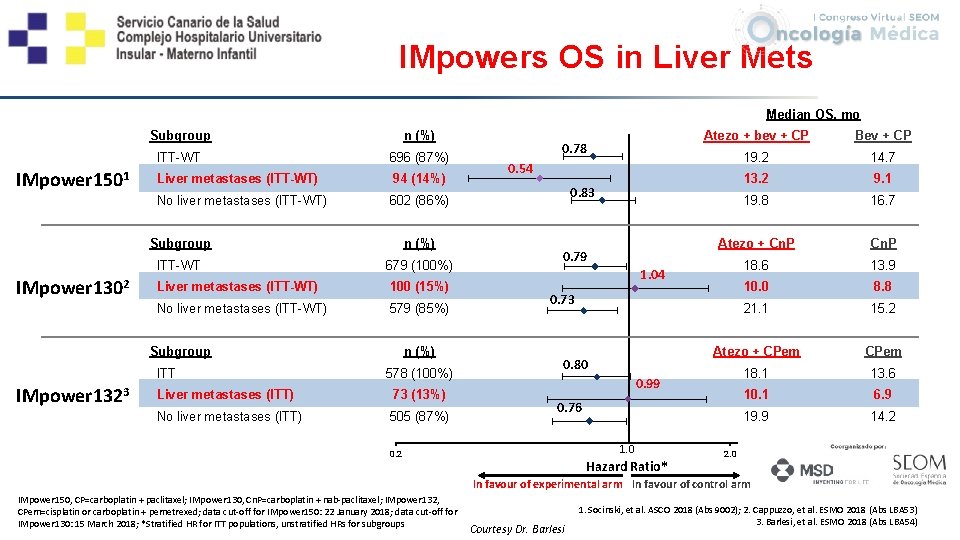
IMpowers OS in Liver Mets Median OS, mo IMpower 1501 Subgroup n (%) ITT-WT 696 (87%) Liver metastases (ITT-WT) 94 (14%) No liver metastases (ITT-WT) 602 (86%) 0. 78 0. 54 0. 83 14 Atezo + bev + CP Bev + CP 19. 2 14. 7 13. 2 9. 1 19. 8 16. 7 Atezo + Cn. P 18. 6 13. 9 10. 0 8. 8 6 21. 1 15. 2 4 Atezo + CPem 18. 1 13. 6 10. 1 6. 9 19. 9 14. 2 12 10 IMpower 1302 Subgroup n (%) ITT-WT 679 (100%) Liver metastases (ITT-WT) 100 (15%) No liver metastases (ITT-WT) 579 (85%) Subgroup ITT IMpower 1323 n (%) 578 (100%) Liver metastases (ITT) 73 (13%) No liver metastases (ITT) 505 (87%) 0. 79 0. 73 0. 80 0. 76 8 0. 99 2 0 0. 2 1. 04 1. 0 Hazard Ratio* 2. 0 In favour of experimental arm In favour of control arm IMpower 150, CP=carboplatin + paclitaxel; IMpower 130, Cn. P=carboplatin + nab-paclitaxel; IMpower 132, CPem=cisplatin or carboplatin + pemetrexed; data cut-off for IMpower 150: 22 January 2018; data cut-off for IMpower 130: 15 March 2018; *Stratified HR for ITT populations, unstratified HRs for subgroups Courtesy Dr. Barlesi 1. Socinski, et al. ASCO 2018 (Abs 9002); 2. Cappuzzo, et al. ESMO 2018 (Abs LBA 53) 3. Barlesi, et al. ESMO 2018 (Abs LBA 54)
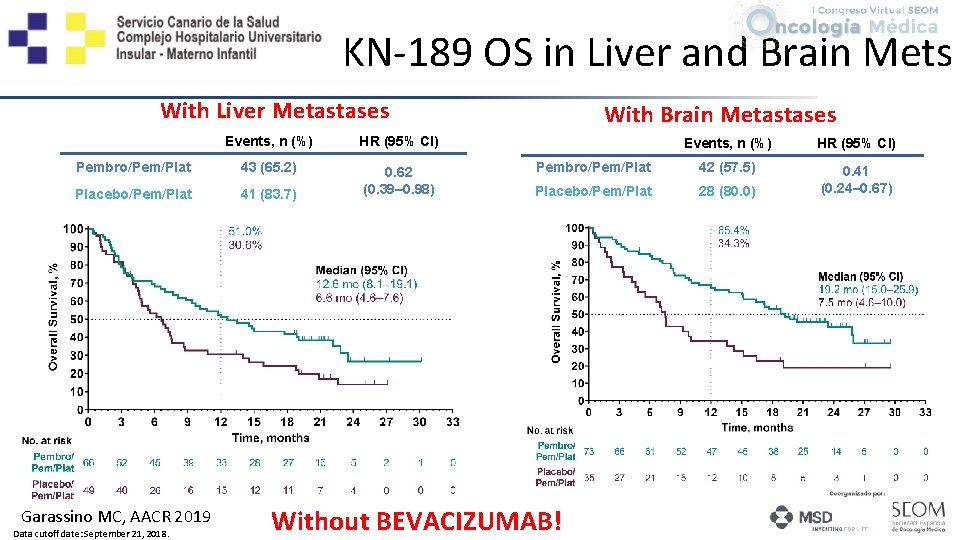
KN-189 OS in Liver and Brain Mets With Liver Metastases Events, n (%) HR (95% CI) Pembro/Pem/Plat 43 (65. 2) Placebo/Pem/Plat 41 (83. 7) 0. 62 (0. 39– 0. 98) Garassino MC, AACR 2019 Data cutoff date: September 21, 2018. With Brain Metastases Events, n (%) HR (95% CI) Pembro/Pem/Plat 42 (57. 5) Placebo/Pem/Plat 28 (80. 0) 0. 41 (0. 24– 0. 67) Without BEVACIZUMAB!
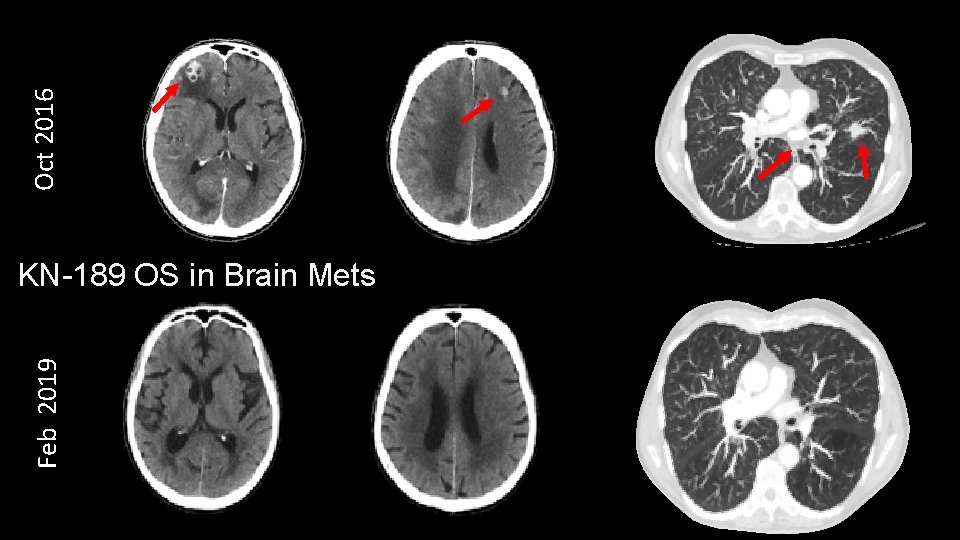
Oct 2016 Feb 2019 KN-189 OS in Brain Mets
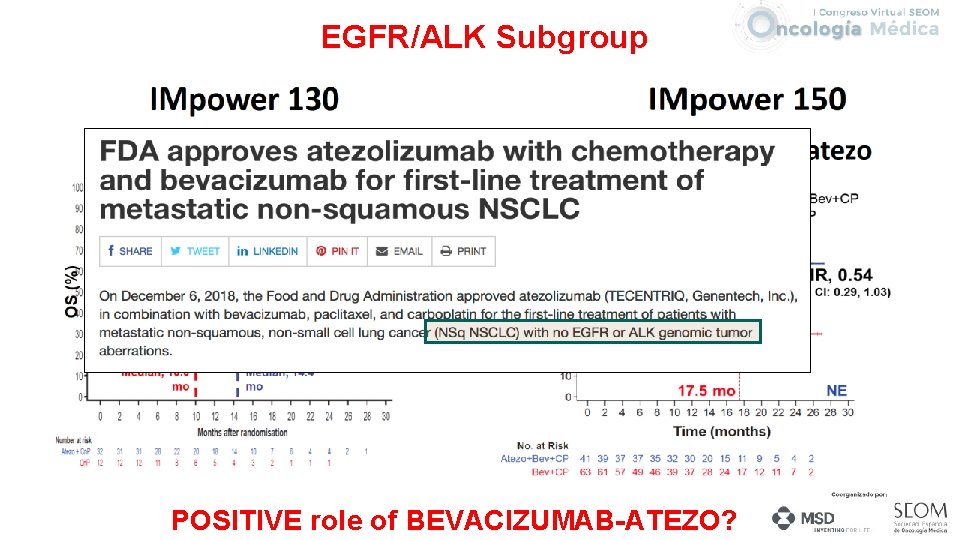
EGFR/ALK Subgroup POSITIVE role of BEVACIZUMAB-ATEZO?

Trying to compare different trials is considere a capital sin but…
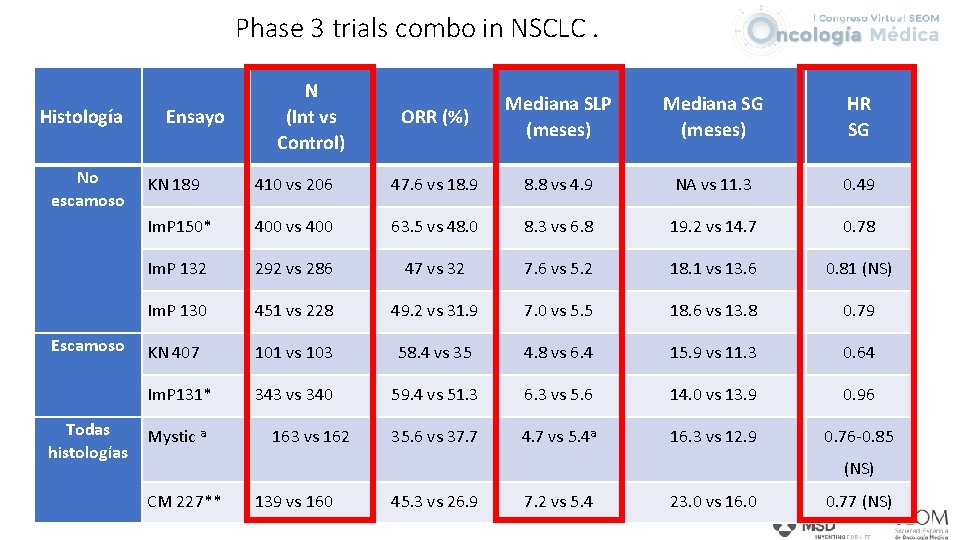
Phase 3 trials combo in NSCLC. Histología No escamoso Escamoso Todas histologías Ensayo N (Int vs Control) ORR (%) Mediana SLP (meses) Mediana SG (meses) HR SG KN 189 410 vs 206 47. 6 vs 18. 9 8. 8 vs 4. 9 NA vs 11. 3 0. 49 Im. P 150* 400 vs 400 63. 5 vs 48. 0 8. 3 vs 6. 8 19. 2 vs 14. 7 0. 78 Im. P 132 292 vs 286 47 vs 32 7. 6 vs 5. 2 18. 1 vs 13. 6 0. 81 (NS) Im. P 130 451 vs 228 49. 2 vs 31. 9 7. 0 vs 5. 5 18. 6 vs 13. 8 0. 79 KN 407 101 vs 103 58. 4 vs 35 4. 8 vs 6. 4 15. 9 vs 11. 3 0. 64 Im. P 131* 343 vs 340 59. 4 vs 51. 3 6. 3 vs 5. 6 14. 0 vs 13. 9 0. 96 35. 6 vs 37. 7 4. 7 vs 5. 4 a 16. 3 vs 12. 9 0. 76 -0. 85 Mystic a 163 vs 162 (NS) CM 227** 139 vs 160 45. 3 vs 26. 9 7. 2 vs 5. 4 23. 0 vs 16. 0 0. 77 (NS)
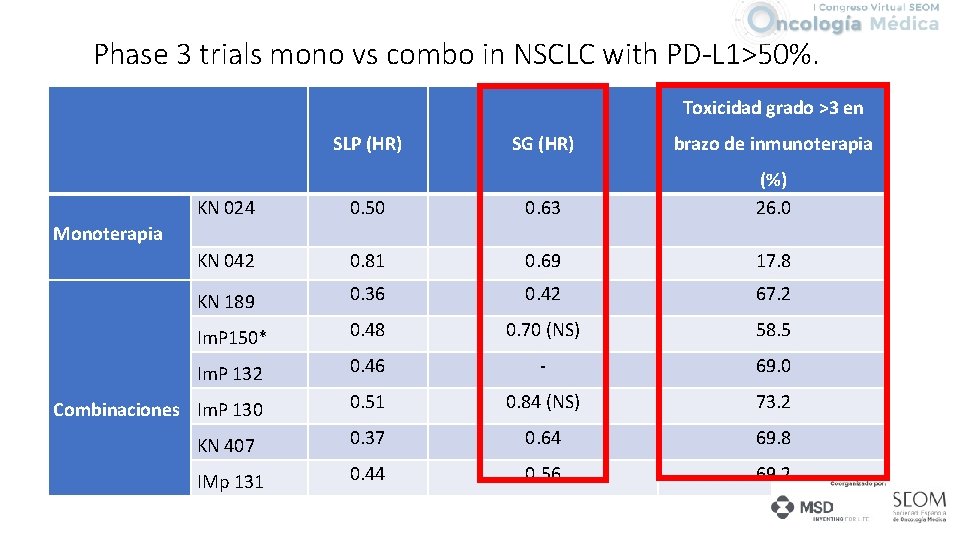
Phase 3 trials mono vs combo in NSCLC with PD-L 1>50%. Toxicidad grado >3 en SLP (HR) SG (HR) brazo de inmunoterapia KN 024 KN 042 0. 50 0. 63 (%) 26. 0 0. 81 0. 69 17. 8 KN 189 0. 36 0. 42 67. 2 Im. P 150* 0. 48 0. 70 (NS) 58. 5 Im. P 132 0. 46 - 69. 0 Combinaciones Im. P 130 0. 51 0. 84 (NS) 73. 2 KN 407 0. 37 0. 64 69. 8 IMp 131 0. 44 0. 56 69. 2 Monoterapia
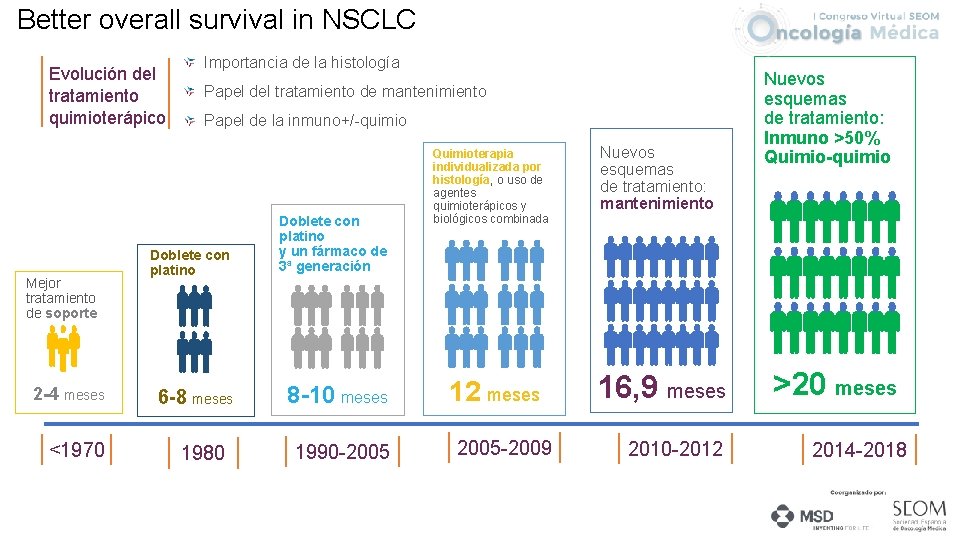
Better overall survival in NSCLC Evolución del tratamiento quimioterápico Mejor tratamiento de soporte 2 -4 meses <1970 Importancia de la histología Papel del tratamiento de mantenimiento Papel de la inmuno+/-quimio Doblete con platino y un fármaco de 3ª generación 6 -8 meses 8 -10 meses 1980 1990 -2005 Quimioterapia individualizada por histología, o uso de agentes quimioterápicos y biológicos combinada 12 meses 2005 -2009 Nuevos esquemas de tratamiento: mantenimiento 16, 9 meses 2010 -2012 Nuevos esquemas de tratamiento: Inmuno >50% Quimio-quimio >20 meses 2014 -2018

Some controversies • PD-L 1>=50% CT-IO or PEMBROLIZUMAB MONO ? • (At 1 y 73% in KN 189 vs 70% KN 024) • (RR 61. 4% in KN 189 vs 44. 8%) • CT plus IO is a NEW STANDARD OF CARE IN 1 L NSCLC. ØIn all PD-L 1 expression ? Yes, but Always better in positive ØIn PD-L 1 negative and low TMB? Need to be demostrate ØWhat is optimal chemotherapy to combine(type, dose, schedule)? • IMpower 150 -Bevacizumab-Atezo: Good results in EGFR, ALK and Liver Mets subgroup? . • We STILL needing betters Biomarkers—How will we use TMB?

THANK YOU!! Dr. Delvys Rodríguez Abreu Hospital Universitario Insular de Gran Canaria. Spain drodabr@gobiernodecanarias. org @delvysra

- Slides: 28