MONOATOMIC VS POLYATOMIC IONS NAMING IONIC FORMULAS Objectives

MONOATOMIC VS. POLYATOMIC IONS NAMING IONIC FORMULAS

Objectives Today I will be able to: Differentiate between monoatomic and polyatomic ions Write ionic formulas using the criss-cross and snap-it together methods Informal assessment – monitoring student interactions and questions as we complete the writing formulas practice Formal assessment – analyzing responses to the practice and exit ticket Common Core Connection Build strong content knowledge Look for and express regularity in repeated reasoning

Lesson Sequence Evaluate: Warm-Up Explore: Monoatomic vs. Polyatomic Ions Explain: Writing Ionic Formulas (Snap-it vs. Criss-Cross) Elaborate: Writing Ionic Formula Practice Evaluate: Exit Ticket
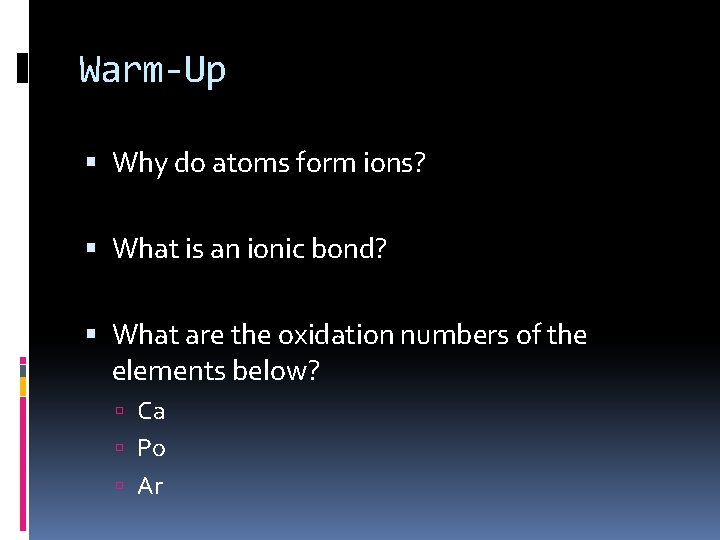
Warm-Up Why do atoms form ions? What is an ionic bond? What are the oxidation numbers of the elements below? Ca Po Ar

Objectives Today I will be able to: Differentiate between monoatomic and polyatomic ions Write ionic formulas using the criss-cross and snap -it together methods

Homework Have a great Thanksgiving break!

Agenda Warm-Up Monatomic vs. Polyatomic Ions Writing Ionic Formulas Snap-It Criss-Cross Writing Ionic Formulas Practice Exit Ticket
Watch Ms. Ose’s demo on the board and take notes. MONOATOMIC VS. POLYATOMIC IONS

Pass out to students PINK SHEET AND POLYATOMIC IONS LIST

WRITING IONIC FORMULAS

Two Methods Snap-It Together Criss-Cross Method

Snap-It Together Na 1+ Cl 1 Na. Cl

Snap-It Together Practice Ca 2+ H+ Al 3+ O 2 IP 3 -

Snap-It Together Answers Ca. O HI Al. P
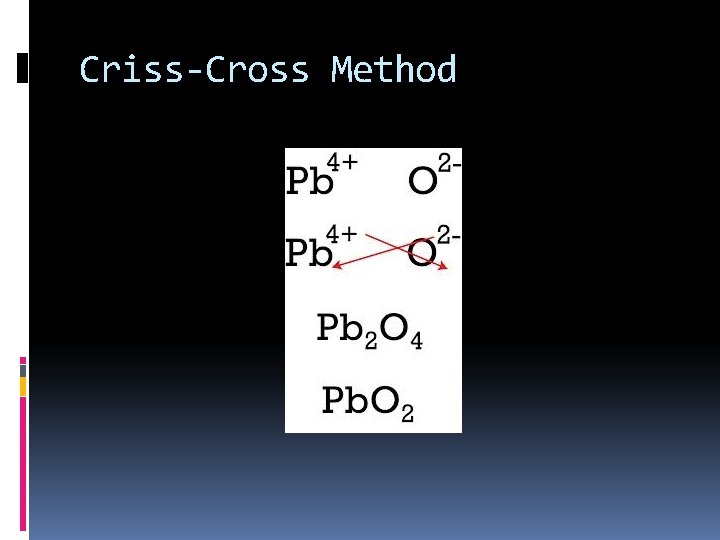
Criss-Cross Method

Criss-Cross Practice Mg 2+ Na+ Fe 3+ Cl. S 2 O 2 -
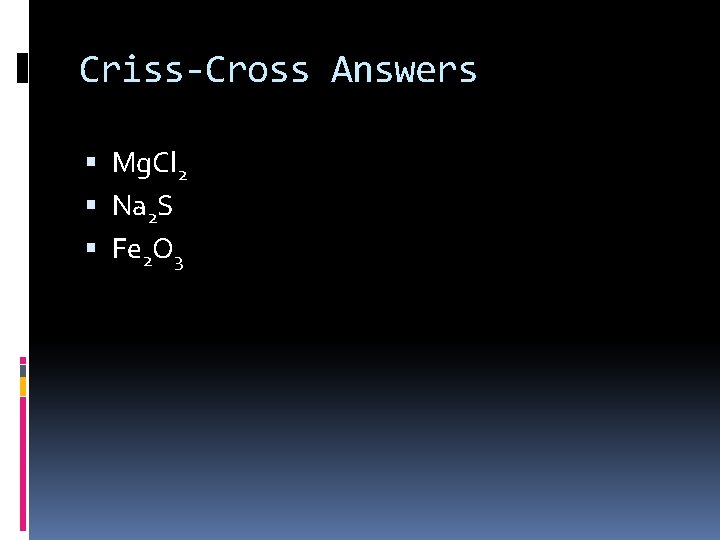
Criss-Cross Answers Mg. Cl 2 Na 2 S Fe 2 O 3

Complete the practice at your desk. Whatever you do not finish will become your homework. WRITING IONIC FORMULAS PRACTICE

Exit Ticket Determine what is wrong in the following ionic formula Al 3 Cl
- Slides: 19