Monitoring thermodynamic equilibrium processes at 10 K Conformational
Monitoring thermodynamic equilibrium processes at 10 K: Conformational isomerization and photochromism of O 4+ in argon matrices Ryan M. Ludwig and David T. Moore Chemistry Department, Lehigh University 69 th ISMS, Univ. Illinois Urbana-Champaign June 17, 2014

Cryogenic spectroscopy kinetic control • Low temperature limit internal energy of system • Cryogenic matrix dissipative medium to remove energy released upon complexation/reaction • Stabilize weakly-bound/transient species “freeze-out” motion on PES before statistical equilibrium can be established (detailed balance) • Thermodynamic chemical equilibrium rare (unprecedented? ), but not impossible 2 requirements • Low barrier (< 5 k. J/mol) • Must not be “sterically challenged”

Counter-ion co-deposition • simult. deposition of mass-selected anions & counter-cations Talk WG 06 • current-matched, lowenergy beams o Cu- & Ar+ o 10 -60 e. V o ~3 -4 n. A for 4 hr o ~1% O 2/Ar @ 20 K o est. ion abundance ~1: 106 ions: Ar • O 4+ ions formed during deposition charge transfer, aggregation • IP: O 2 ~12. 1 e. V, atomic Ar ~15. 7 e. V, solid Ar ~13. 9 e. V Ludwig R. ; DTM, J. Chem. Phys. 139, 244202 (2013). Zhou, M. ; Hacaloglu, J. ; Andrews, L. , J. Chem. Phys. 110, 9450 (1999).

O 4+ in Ar at 10 K • trans- & cyc-O 4+ Jacox (neon), Andrews (argon) 18 O • assignments based on theory cyc. ex. state (~0. 5 kcal/mol) 2 • Cu- charge balance only O 4 - • 300 e. V Ar+ (unbalanced) 300 e. V Ar+ (O 2)Cu(O 2) trans-O 4+ • strong O 4 - peak cyc-O 4+ (trans-O 4+)---(O 2) Zhou, M. ; Hacaloglu, J. ; Andrews, L. , J. Chem. Phys. 110, 9450 (1999). Jacox, M. E. ; Thompson, W. E. , J. Chem. Phys. 100, 750 (1994). cyc-O 6+ • 18 O 2 all bands exhibit expected shifts Lindh, R. ; Barnes, L. A. , J. Chem. Phys. 100, 224 (1994).

Temperature dependence of O 4+ bands 10 K • trans-O 4+ dec. w/inc. T new band grows in near 1310 cm-1 16 K 15 K • REVERSIBLE(!!) exchanging populations ? 14 K 13 K • lin. relationship between band intensities 12 K 11 K 10 K wavenumber (cm-1) Itot = s. AXA + s. BXB; XA + XB = 1 • 18 O 4+ same behavior for analogous bands • all consistent w/ conformational equilibrium

Temperature dependence of O 4+ bands • irreversible loss of trans. O 4+ upon heating above 22 K • correlated disappearance of 1310 cm-1 band • no band growth loss to “dark” species O 2+ & O 2 • equilibrating O 4+ system kinetically trapped • cryogenic synthesis of metastable intermediate wavenumber (cm-1) O 2 + O 2+ trans cyc O 2 + O 2+
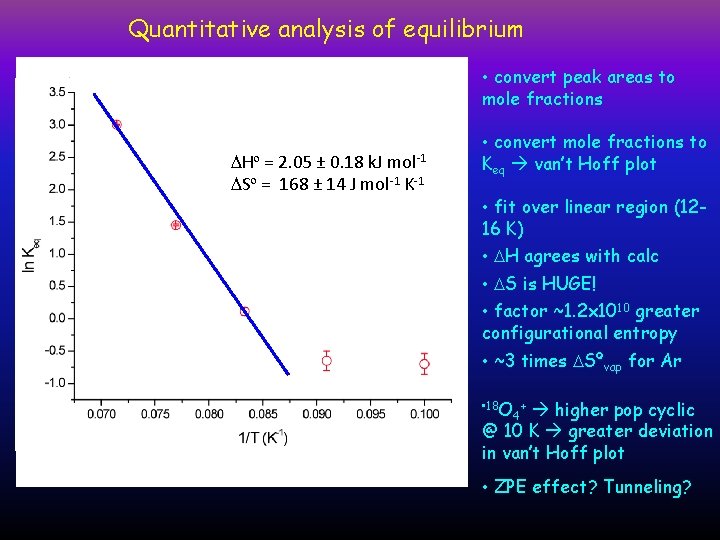
Quantitative analysis of equilibrium Mole Fraction • convert peak areas to mole fractions Keq DHº = 2. 05 ± 0. 18 k. J DSº = =168 ± 14 J mol K-1 -1 DHº 2. 46 ± 0. 04 k. J-1 mol DSº = 193 ± 3 J mol-1 K-1 mol-1 T (K) • convert mole fractions to Keq van’t Hoff plot • fit over linear region (1216 K) • DH agrees with calc • DS is HUGE! • factor ~1. 2 x 1010 greater configurational entropy • ~3 times DSºvap for Ar • 18 O + 4 higher pop cyclic @ 10 K greater deviation in van’t Hoff plot • ZPE effect? Tunneling?

“Cavity-coupling” model • Equilibrating system must include some Ar • Double substitution site in fcc lattice of solid Ar • trans-O 4+ fits almost perfectly, “static” • cyclic-O 4+ more compact, “mobile” • DS trend qualitatively correct larger cavity? trans cyclic

Photo-isomerization of O 4+ species All spectra taken at 10 K • “trans-O 6+” affected by visible irradiation • 590 nm small enh. • 740 nm depletion • 470 nm large enh. • REVERSIBLE changes • 1186/1331 cm-1 pair in equil. @ 10 K • BD/6 -311+G(3 df) calcs • C 2 h doub: 795 cm-1 quart: 887 cm-1 • D 2 h doub: 1166 cm-1 quart: 1191 cm-1 • DEdoub-quart = ~ 50 k. J/mol

Conclusions • thermodynamic chemical equilibrium observed at 10 K between conformers of O 4+ • irreversible loss of equlibrating pair above 22 K kinetically trapped intermediates • reaction coordinate involves coupling of the ion motion to matrix cavity • another pair of O 4+ conformers exhibits reversible photochromism at 10 K • at least 5 distinct conformational/electronic states are trapped during matrix formation mechanism? ? • GOOD THEORY IS DESPERATELY NEEDED!!!

Deposition at 25 K

Ar+ only (no Cu-) deposition at 20 K

Acknowledgements Moore Group § Angela Smith § Nina Finamore § Ryan Ludwig § Mike Goodrich § Becky Klimas (M. S. ) § Alex Hunter (M. S. ) undergraduates § Edric Miro Gavin § Erin Hassell §Tony Thompson §Christina Marrone §Chris Caputo §Nick Greybush §Ankit Pokherel §Nick Voellinger Funding §NSF CAREER Award §Lehigh Univ. (Start-Up)

Cu- with 0. 5% O 2 (Kr+ counter ion) • spectrum dominated by (O 2)n+ peaks, no anions evident • only copper species is weak band assigned to Cu(O 2)2 in literature
- Slides: 14