Monitoring in vitro human digestion of model food
- Slides: 1

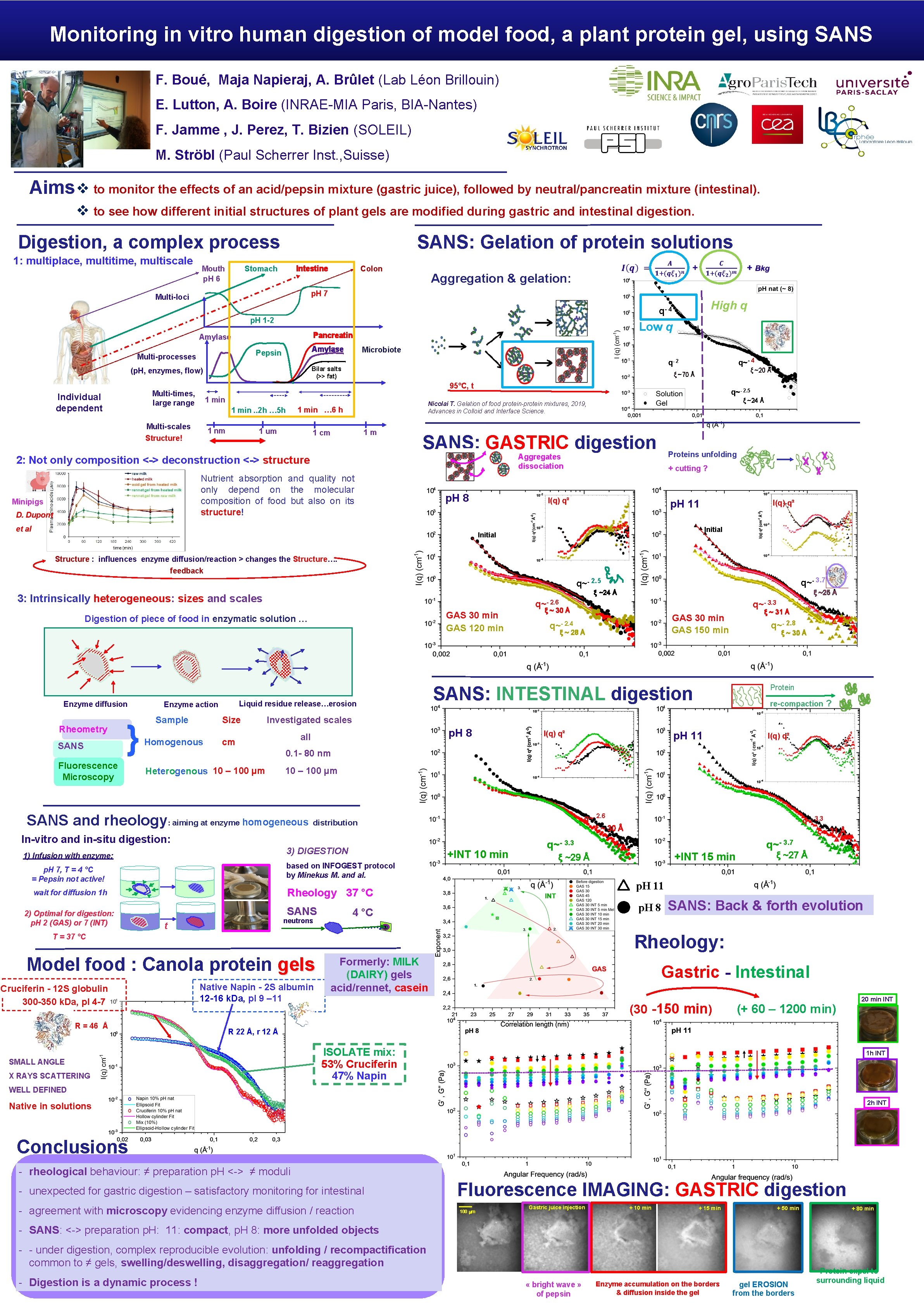
Monitoring in vitro human digestion of model food, a plant protein gel, using SANS F. Boué, Maja Napieraj, A. Brûlet (Lab Léon Brillouin) E. Lutton, A. Boire (INRAE-MIA Paris, BIA-Nantes) F. Jamme , J. Perez, T. Bizien (SOLEIL) M. Ströbl (Paul Scherrer Inst. , Suisse) Aims v to monitor the effects of an acid/pepsin mixture (gastric juice), followed by neutral/pancreatin mixture (intestinal). v to see how different initial structures of plant gels are modified during gastric and intestinal digestion. Digestion, a complex process 1: multiplace, multitime, multiscale Mouth p. H 6 SANS: Gelation of protein solutions Stomach Intestine Colon Aggregation & gelation: p. H 7 Multi-loci High q q- 4 p. H 1 -2 Pancreatin Amylase Pepsin Multi-processes Microbiote 1 min 1 nm 1 um q~- 2. 5 ξ ~24 Å Nicolai T. Gelation of food protein-protein mixtures, 2019, Advances in Colloid and Interface Science. 1 min … 6 h 1 min. . 2 h … 5 h Multi-scales Structure! 1 m 1 cm SANS: GASTRIC digestion Proteins unfolding Aggregates dissociation 2: Not only composition <-> deconstruction <-> structure Nutrient absorption and quality not only depend on the molecular composition of food but also on its structure! D. Dupont ξ ~20 Å ξ ~70 Å 95°C, t Multi-times, large range Minipigs q~- 4 q- 2 Bilar salts (>> fat) (p. H, enzymes, flow) Individual dependent Low q p. H 8 et al + cutting ? I(q) q² p. H 11 I(q) q² Initial Structure : influences enzyme diffusion/reaction > changes the Structure…. feedback q~- 3. 7 q~- 2. 5 3: Intrinsically heterogeneous: sizes and scales q~- 2. 6 GAS 30 min GAS 120 min Digestion of piece of food in enzymatic solution … Enzyme diffusion Rheometry SANS } Fluorescence Microscopy Liquid residue release…erosion Enzyme action Sample Size Homogenous q~- 3. 3 ξ ~ 30 Å ξ ~ 31 Å GAS 30 min GAS 150 min q~- 2. 4 ξ ~ 28 Å q~- 2. 8 ξ ~ 30 Å Protein SANS: INTESTINAL digestion re-compaction ? Investigated scales p. H 8 all cm I(q) q² p. H 11 I(q) q² 0. 1 - 80 nm Heterogenous 10 – 100 µm SANS and rheology: aiming at enzyme homogeneous q~- 2. 6 ξ ~ 30 Å distribution In-vitro and in-situ digestion: 3) DIGESTION 1) Infusion with enzyme: +INT 10 min p. H 7, T = 4 °C = Pepsin not active! based on INFOGEST protocol by Minekus M. and al. wait for diffusion 1 h Rheology 37 °C 2) Optimal for digestion: p. H 2 (GAS) or 7 (INT) ξ ~25 Å ξ ~24 Å SANS neutrons t q~- 3. 3 q~- 3. 7 ξ ~27 Å p. H 11 p. H 8 4 °C SANS: Back & forth evolution Rheology: Model food : Canola protein gels R = 46 Å +INT 15 min ξ ~29 Å T = 37 °C Cruciferin - 12 S globulin ~hollow cylinder: core= 101 Å, R = 46+-3Å , L = 52+-3Å 300 -350 k. Da, p. I 4 -7 q~- 3. 3 ξ ~ 31 Å Native Napin - 2 S albumin 12 -16 k. Da, p. I 9 – 11 Formerly: MILK (DAIRY) gels acid/rennet, casein Gastric - Intestinal (30 -150 min) (+ 60 – 1200 min) 20 min INT R 22 Å, r 12 Å SMALL ANGLE X RAYS SCATTERING ISOLATE mix: 53% Cruciferin 47% Napin 1 h INT WELL DEFINED 2 h INT Native in solutions Conclusions - rheological behaviour: ≠ preparation p. H <-> ≠ moduli - unexpected for gastric digestion – satisfactory monitoring for intestinal - agreement with microscopy evidencing enzyme diffusion / reaction Fluorescence IMAGING: GASTRIC digestion 100 mm Gastric juice injection + 10 min + 15 min + 50 min + 80 min - SANS: <-> preparation p. H: 11: compact, p. H 8: more unfolded objects - - under digestion, complex reproducible evolution: unfolding / recompactification common to ≠ gels, swelling/deswelling, disaggregation/ reaggregation - Digestion is a dynamic process ! « bright wave » of pepsin Enzyme accumulation on the borders & diffusion inside the gel EROSION from the borders Protein expel to surrounding liquid