Monday 111918 1 Please have these Items on




- Slides: 9

Monday 11/19/18 1. Please have these Items on your desk. Day 1 Science Starters Sheet Agenda Super Hero Element 2 - Fill out your Agenda. • What did you enjoy about the superhero activity? • What does the atomic mass tell us about an element?

Table of contents update Page 69 11/16 Bonding Basics Notes

8 th Grade Science T. Trimpe 2008 http: //sciencespot. net/

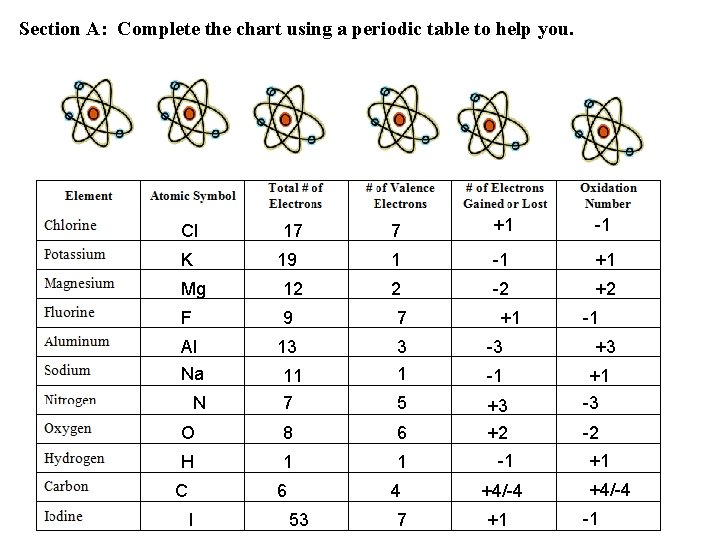
Section A: Complete the chart using a periodic table to help you. Cl K 17 19 7 +1 -1 +1 2 -2 +2 Mg 12 F 9 7 Al 13 3 -3 11 7 1 -1 5 O 8 6 +3 +2 H 1 1 Na N C 6 I 4 53 7 +1 -1 +4/-4 +1 -1 +3 +1 -3 -2 +1 +4/-4 -1

Answer these questions: NEGATIVE An atom that gains one or more electrons will have a __________ charge. POSITIVE An atom that loses one or more electrons will have a __________ charge. ION An atom that gains or loses one or more electrons is called an ______. CATION and a negative ion is called an A positive ion is called a _______ ANION ________. “Cat-Eye-On” “An-Eye-On”

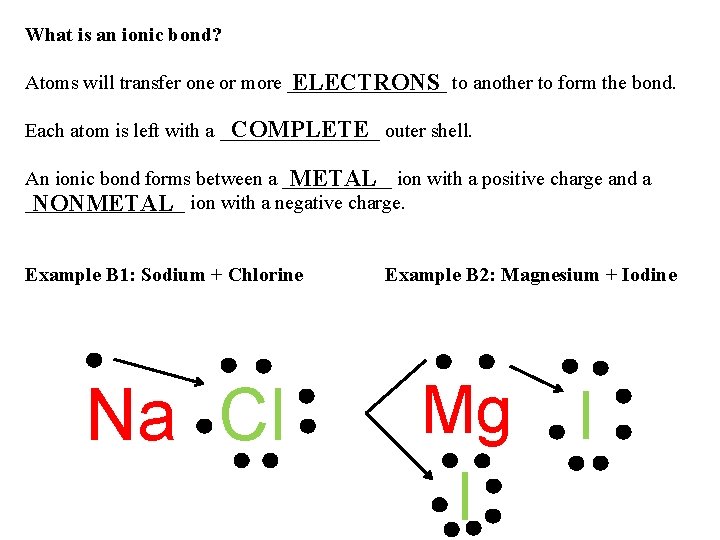
What is an ionic bond? Atoms will transfer one or more ________ ELECTRONS to another to form the bond. COMPLETE outer shell. Each atom is left with a ________ An ionic bond forms between a ______ METAL ion with a positive charge and a ________ NONMETAL ion with a negative charge. Example B 1: Sodium + Chlorine Na Cl Example B 2: Magnesium + Iodine Mg I I

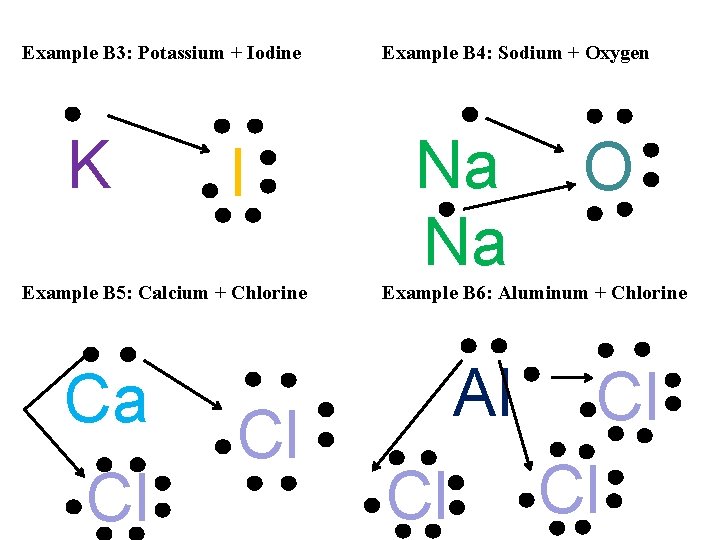
Example B 3: Potassium + Iodine K I Example B 5: Calcium + Chlorine Ca Cl Cl Example B 4: Sodium + Oxygen Na Na O Example B 6: Aluminum + Chlorine Al Cl Cl Cl

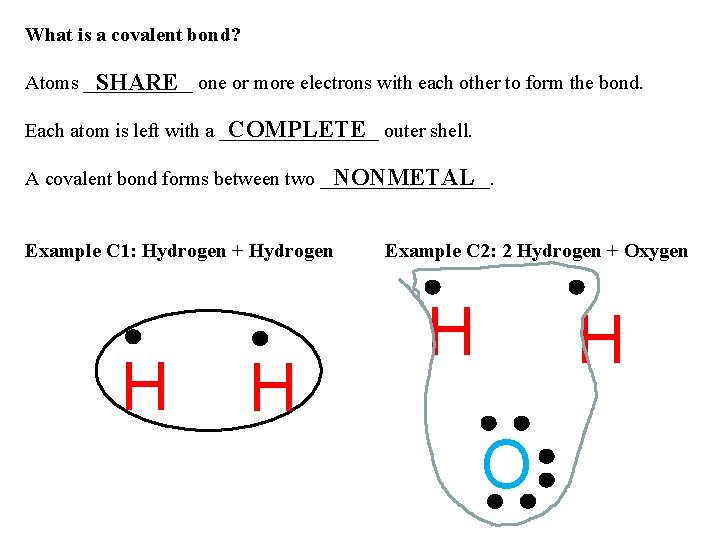
What is a covalent bond? Atoms ______ SHARE one or more electrons with each other to form the bond. COMPLETE outer shell. Each atom is left with a ________ NONMETAL A covalent bond forms between two _________. Example C 1: Hydrogen + Hydrogen H H Example C 2: 2 Hydrogen + Oxygen H H O

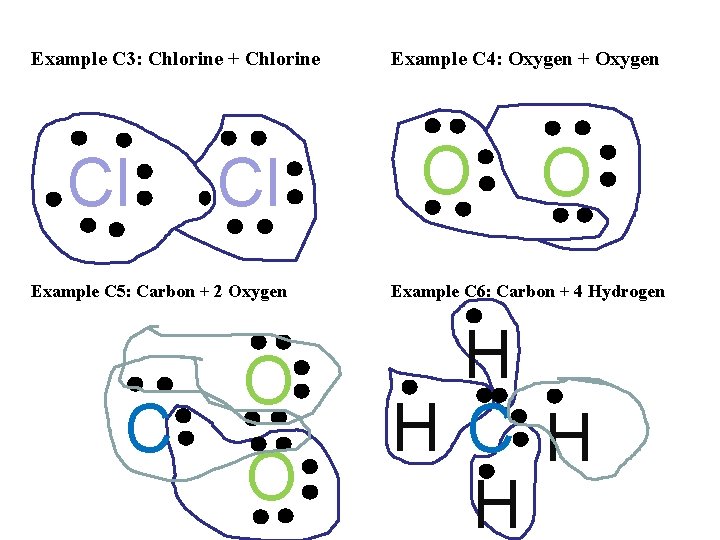
Example C 3: Chlorine + Chlorine Cl Cl Example C 5: Carbon + 2 Oxygen C O O Example C 4: Oxygen + Oxygen O O Example C 6: Carbon + 4 Hydrogen H H C H H