MOLES TO GRAMS CONVERSIONS AND COMPOSITION Molar Mass


- Slides: 16

MOLES TO GRAMS CONVERSIONS AND % COMPOSITION

Molar Mass The mass in grams of 1 mole of a substance is called the molar mass of the substance. The molar mass of an element is equal to its atomic mass. The unit for molar mass is grams per mole, or g/mol

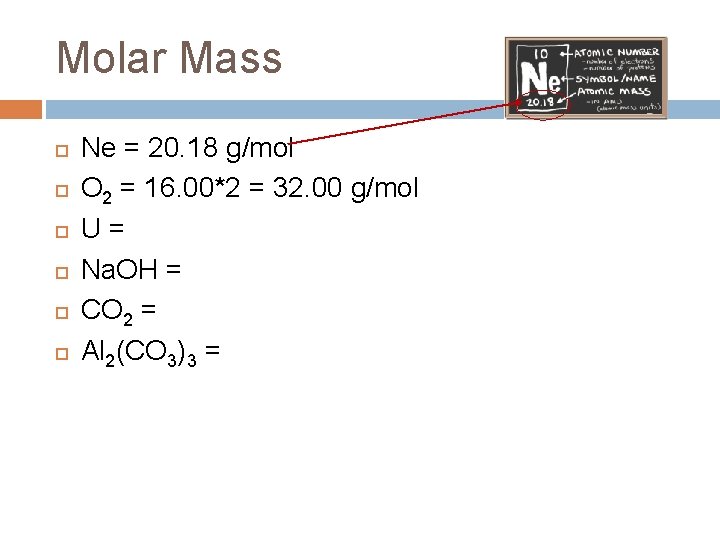
Molar Mass Ne = 20. 18 g/mol O 2 = 16. 00*2 = 32. 00 g/mol U = 238. 03 g/mol Na. OH = 22. 99 + 16. 00 + 1. 008 = 40. 00 g/mol CO 2 = 12. 01 + 16. 00*2 = 44. 01 g/mol Al 2(CO 3)3 = 26. 98*2 + (12. 01*3) + (16. 00*9) = 233. 99 g/mol

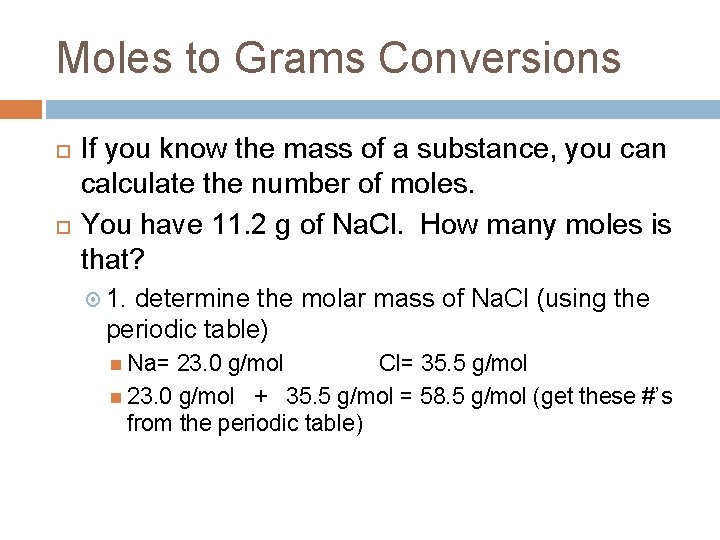
Moles to Grams Conversions If you know the mass of a substance, you can calculate the number of moles. You have 11. 2 g of Na. Cl. How many moles is that? 1. determine the molar mass of Na. Cl (using the periodic table) Na= 23. 0 g/mol Cl= 35. 5 g/mol 23. 0 g/mol + 35. 5 g/mol = 58. 5 g/mol (get these #’s from the periodic table)

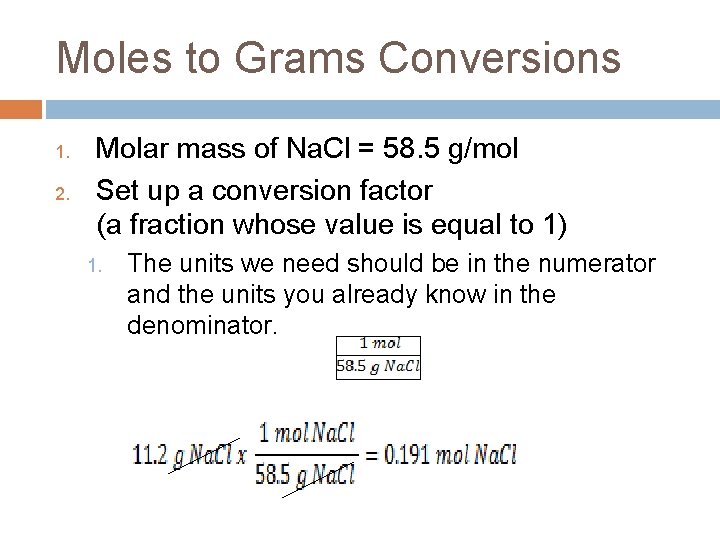
Moles to Grams Conversions 1. 2. Molar mass of Na. Cl = 58. 5 g/mol Set up a conversion factor (a fraction whose value is equal to 1) 1. The units we need should be in the numerator and the units you already know in the denominator.

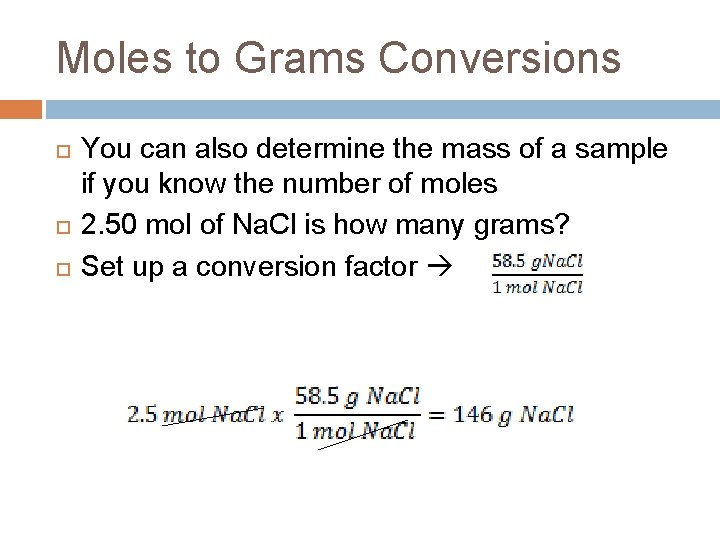
Moles to Grams Conversions You can also determine the mass of a sample if you know the number of moles 2. 50 mol of Na. Cl is how many grams? Set up a conversion factor

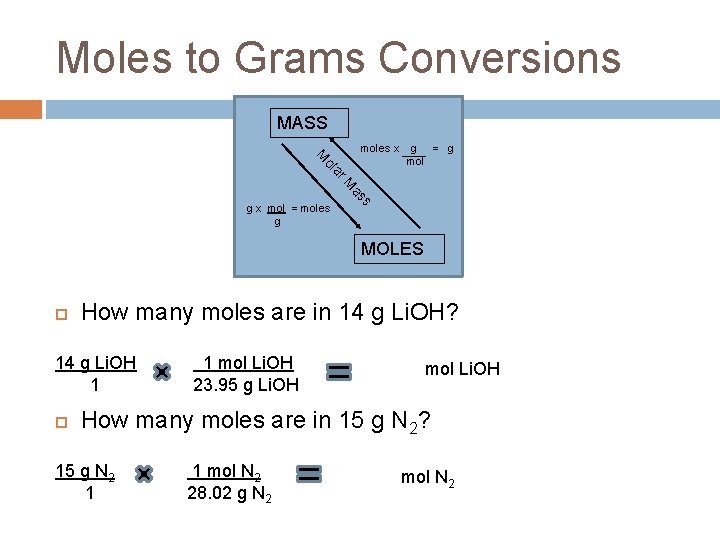
Moles to Grams Conversions MASS moles x ar ol M g = g mol s as M g x mol = moles g MOLES How many moles are in 14 g Li. OH? 14 g Li. OH 1 1 mol Li. OH 23. 95 g Li. OH 0. 58 mol Li. OH How many moles are in 15 g N 2? 15 g N 2 1 1 mol N 2 28. 02 g N 2 0. 54 mol N 2

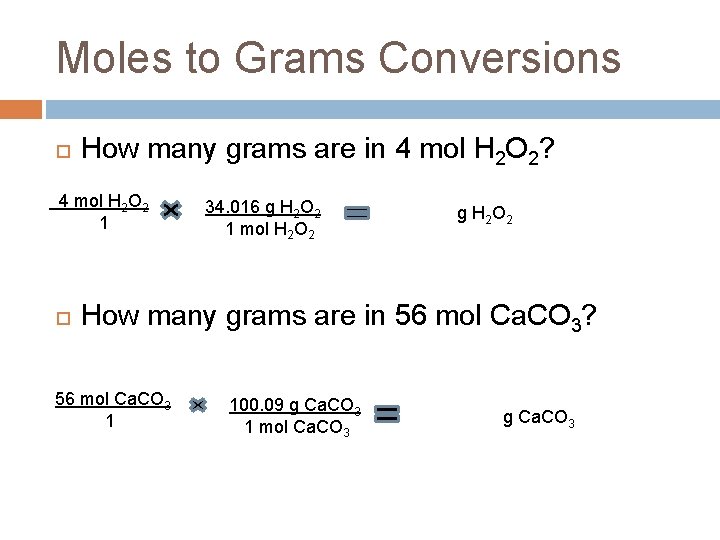
Moles to Grams Conversions How many grams are in 4 mol H 2 O 2? 4 mol H 2 O 2 1 34. 016 g H 2 O 2 1 mol H 2 O 2 136. 06 g H 2 O 2 How many grams are in 56 mol Ca. CO 3? 56 mol Ca. CO 3 1 100. 09 g Ca. CO 3 1 mol Ca. CO 3 5605. 04 g Ca. CO 3

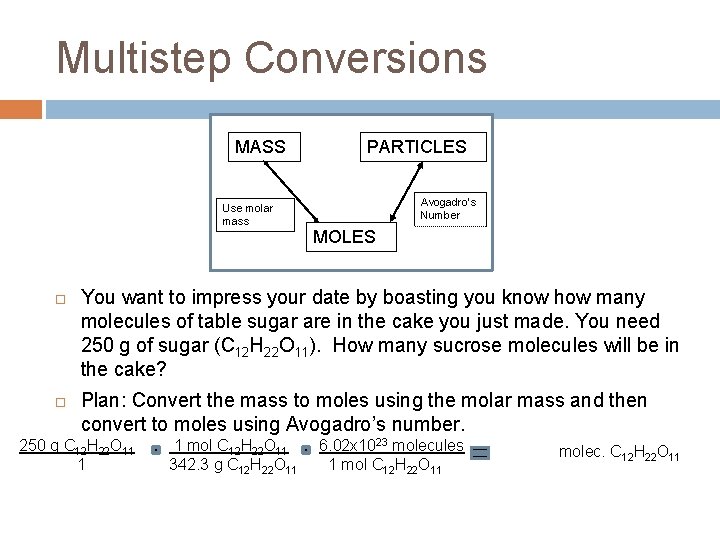
Multistep Conversions MASS Use molar mass PARTICLES Avogadro’s Number MOLES You want to impress your date by boasting you know how many molecules of table sugar are in the cake you just made. You need 250 g of sugar (C 12 H 22 O 11). How many sucrose molecules will be in the cake? Plan: Convert the mass to moles using the molar mass and then convert to moles using Avogadro’s number. 250 g C 12 H 22 O 11 1 1 mol C 12 H 22 O 11 342. 3 g C 12 H 22 O 11 6. 02 x 1023 molecules 1 mol C 12 H 22 O 11 4. 4 x 1023 molec. C 12 H 22 O 11

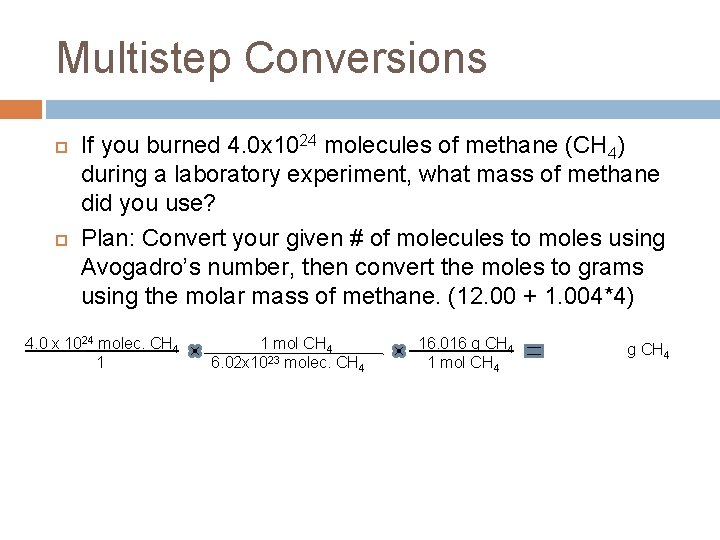
Multistep Conversions If you burned 4. 0 x 1024 molecules of methane (CH 4) during a laboratory experiment, what mass of methane did you use? Plan: Convert your given # of molecules to moles using Avogadro’s number, then convert the moles to grams using the molar mass of methane. (12. 00 + 1. 004*4) 4. 0 x 1024 molec. CH 4 1 1 mol CH 4 6. 02 x 1023 molec. CH 4 16. 016 g CH 4 1 mol CH 4 106. 42 g CH 4

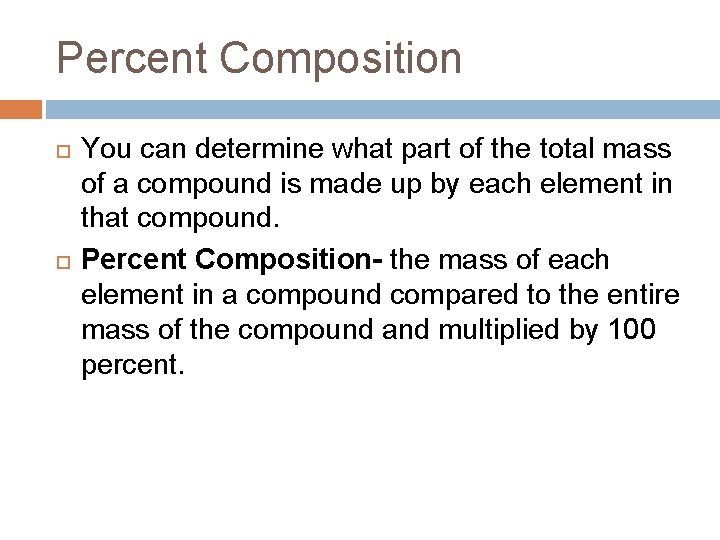
Percent Composition You can determine what part of the total mass of a compound is made up by each element in that compound. Percent Composition- the mass of each element in a compound compared to the entire mass of the compound and multiplied by 100 percent.

Determining % Composition First method: Calculate from a given formula Example: Water 1 mole of water (H 2 O) Molar mass= 18 grams 2 moles of hydrogen atoms 1 mole of oxygen atoms To determine percent composition you need to determine what part of the total mass, 18 g, is made up of hydrogen atoms and what part is made up of oxygen atoms.

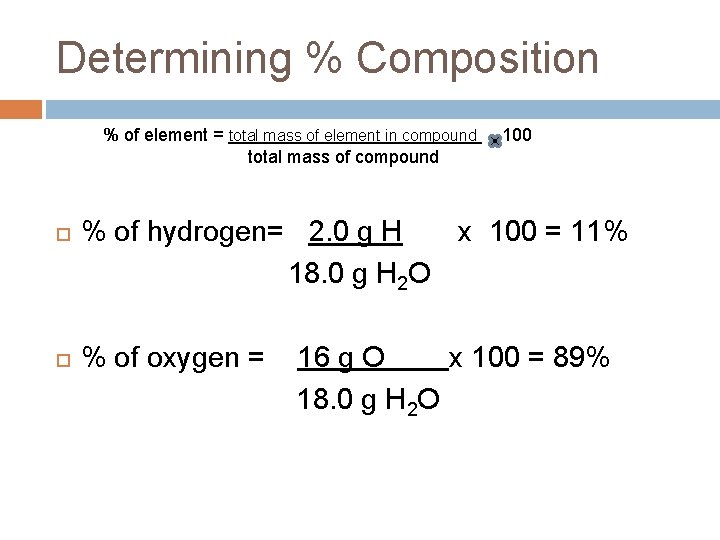
Determining % Composition % of element = total mass of element in compound total mass of compound 100 % of hydrogen= 2. 0 g H x 100 = 11% 18. 0 g H 2 O % of oxygen = 16 g O x 100 = 89% 18. 0 g H 2 O

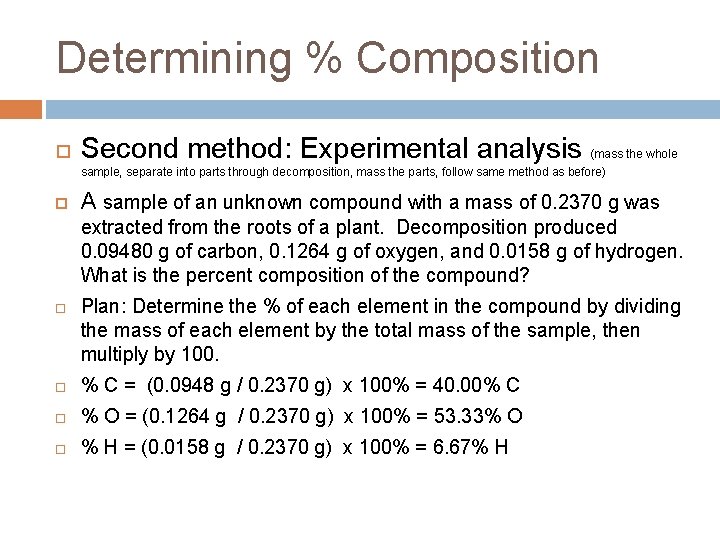
Determining % Composition Second method: Experimental analysis (mass the whole sample, separate into parts through decomposition, mass the parts, follow same method as before) A sample of an unknown compound with a mass of 0. 2370 g was extracted from the roots of a plant. Decomposition produced 0. 09480 g of carbon, 0. 1264 g of oxygen, and 0. 0158 g of hydrogen. What is the percent composition of the compound? Plan: Determine the % of each element in the compound by dividing the mass of each element by the total mass of the sample, then multiply by 100. % C = (0. 0948 g / 0. 2370 g) x 100% = 40. 00% C % O = (0. 1264 g / 0. 2370 g) x 100% = 53. 33% O % H = (0. 0158 g / 0. 2370 g) x 100% = 6. 67% H

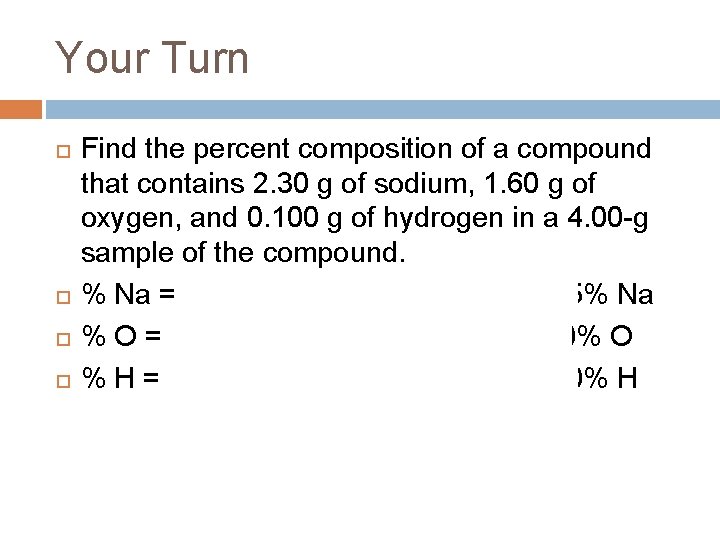
Your Turn Find the percent composition of a compound that contains 2. 30 g of sodium, 1. 60 g of oxygen, and 0. 100 g of hydrogen in a 4. 00 -g sample of the compound. % Na = (2. 30 g / 4. 00 g) x 100% = 57. 5% Na % O = (1. 60 g / 4. 00 g) x 100% = 40. 0% O % H = (0. 100 g / 4. 00 g) x 100% = 2. 50% H

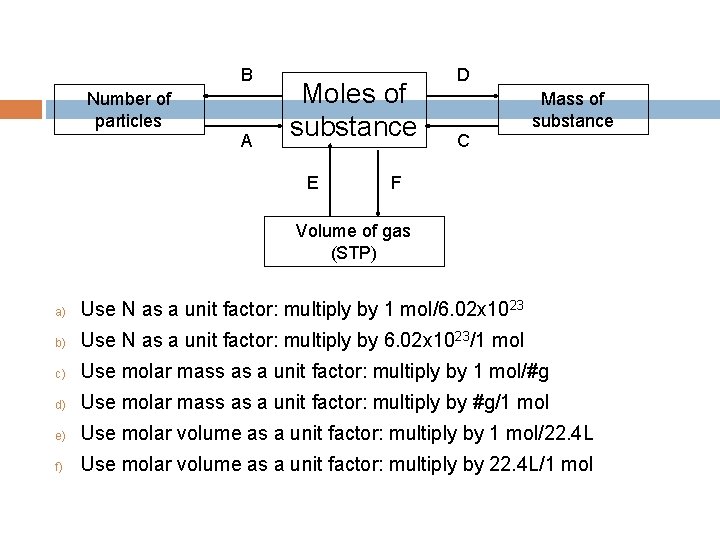
B Number of particles A Moles of substance E D Mass of substance C F Volume of gas (STP) a) Use N as a unit factor: multiply by 1 mol/6. 02 x 1023 b) Use N as a unit factor: multiply by 6. 02 x 1023/1 mol c) Use molar mass as a unit factor: multiply by 1 mol/#g d) Use molar mass as a unit factor: multiply by #g/1 mol e) Use molar volume as a unit factor: multiply by 1 mol/22. 4 L f) Use molar volume as a unit factor: multiply by 22. 4 L/1 mol