Moles and Molarity Practice Problems THE MOLE 1

Moles and Molarity Practice Problems

THE MOLE • 1. What is a MOLE? • The MASS in GRAMS of a substance containing AVAGADRO’S NUMBER of particles (atoms, molecules, etc. ) • Abbreviated mol • 2. The MASS, in GRAMS, of ONE MOLE of any substance is called FORMULA WEIGHT or MOLAR MASS. • The unit is g/mol.
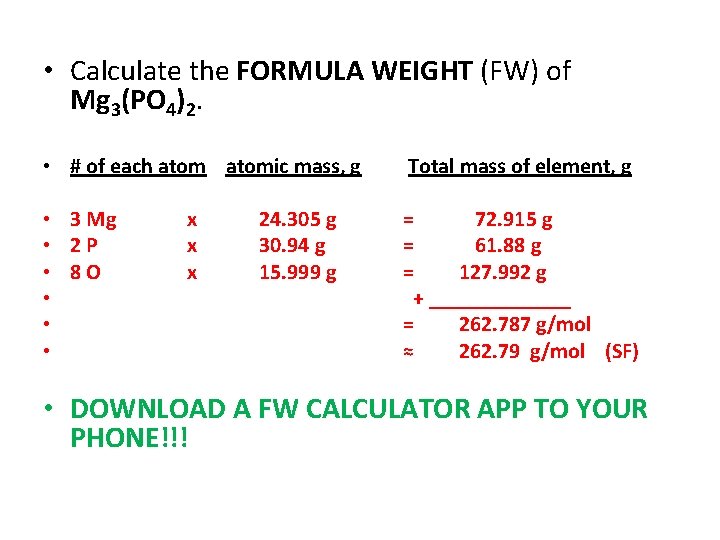
• Calculate the FORMULA WEIGHT (FW) of Mg 3(PO 4)2. • # of each atomic mass, g Total mass of element, g • 3 Mg • 2 P • 8 O • • • = 72. 915 g = 61. 88 g = 127. 992 g + _______ = 262. 787 g/mol ≈ 262. 79 g/mol (SF) x x x 24. 305 g 30. 94 g 15. 999 g • DOWNLOAD A FW CALCULATOR APP TO YOUR PHONE!!!
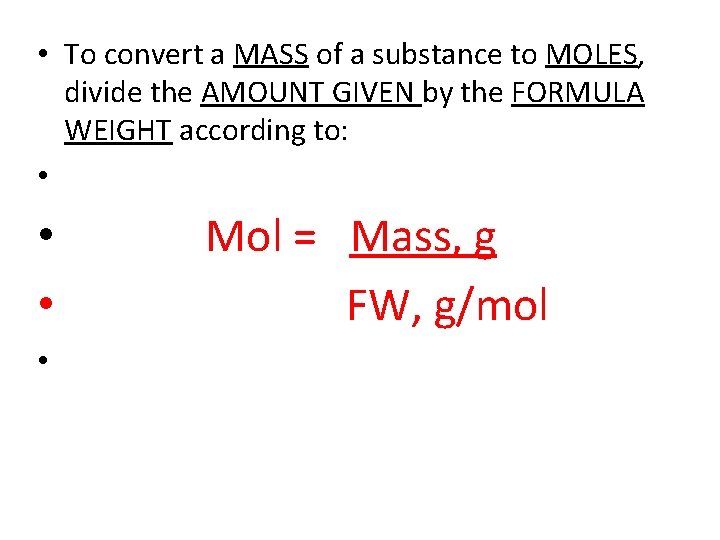
• To convert a MASS of a substance to MOLES, divide the AMOUNT GIVEN by the FORMULA WEIGHT according to: • • Mol = Mass, g FW, g/mol
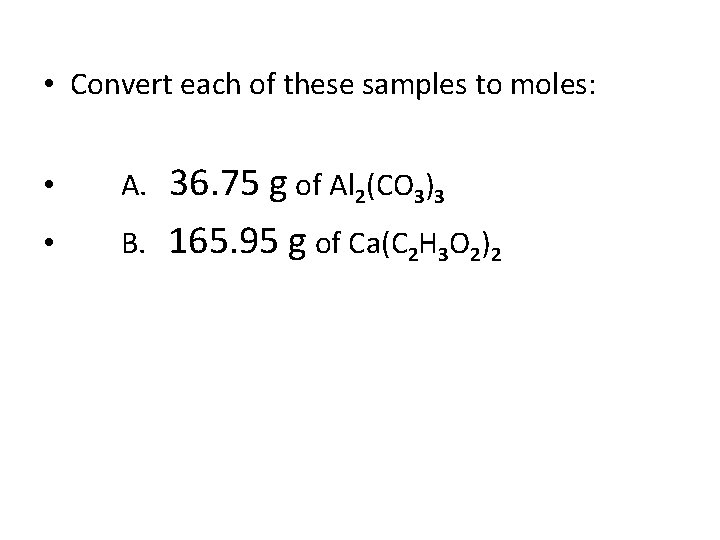
• Convert each of these samples to moles: • A. • B. 36. 75 g of Al 2(CO 3)3 165. 95 g of Ca(C 2 H 3 O 2)2
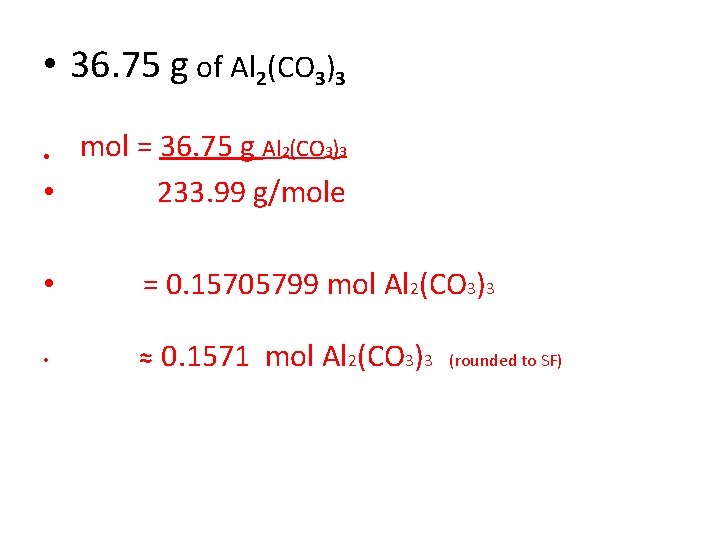
• 36. 75 g of Al 2(CO 3)3 mol = 36. 75 g Al 2(CO 3)3 • 233. 99 g/mole • • = 0. 15705799 mol Al 2(CO 3)3 • ≈ 0. 1571 mol Al 2(CO 3)3 (rounded to SF)

• 165. 95 g of Ca(C 2 H 3 O 2)2 • Mol = 165. 95 g Ca(C 2 H 3 O 2)2 • 158. 17 g/mole • = 1. 049187 mol Ca(C 2 H 3 O 2)2 • ≈ 1. 0492 mol Ca(C 2 H 3 O 2)2
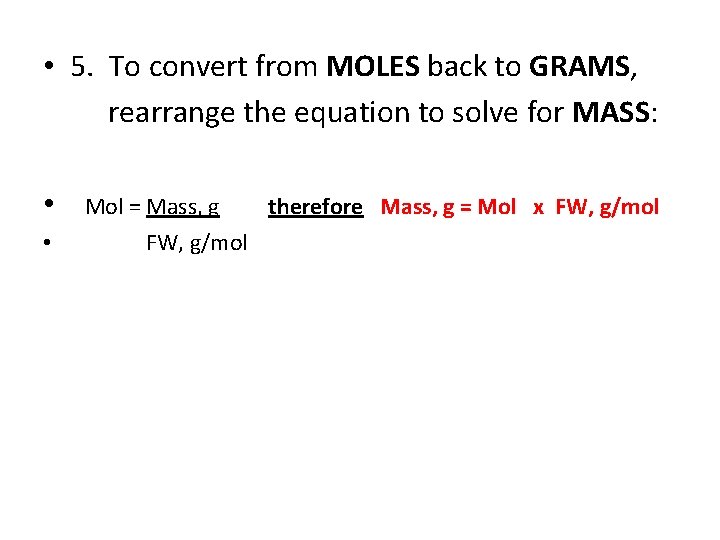
• 5. To convert from MOLES back to GRAMS, rearrange the equation to solve for MASS: • • Mol = Mass, g FW, g/mol therefore Mass, g = Mol x FW, g/mol
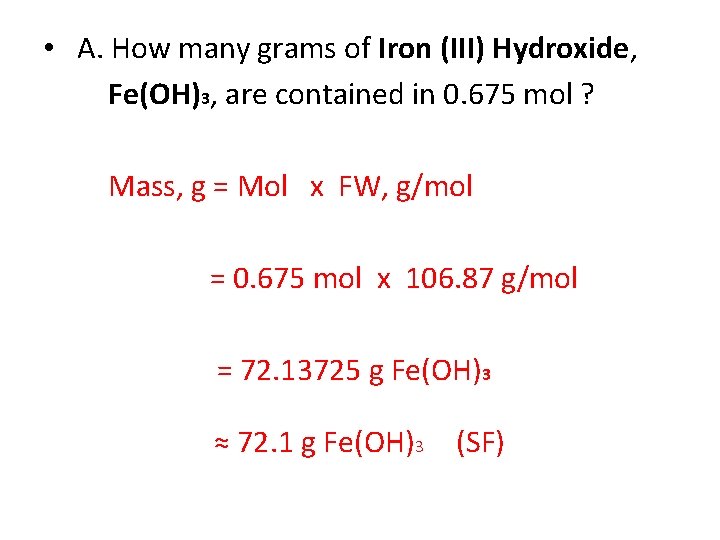
• A. How many grams of Iron (III) Hydroxide, Fe(OH)3, are contained in 0. 675 mol ? Mass, g = Mol x FW, g/mol = 0. 675 mol x 106. 87 g/mol = 72. 13725 g Fe(OH)3 ≈ 72. 1 g Fe(OH)3 (SF)
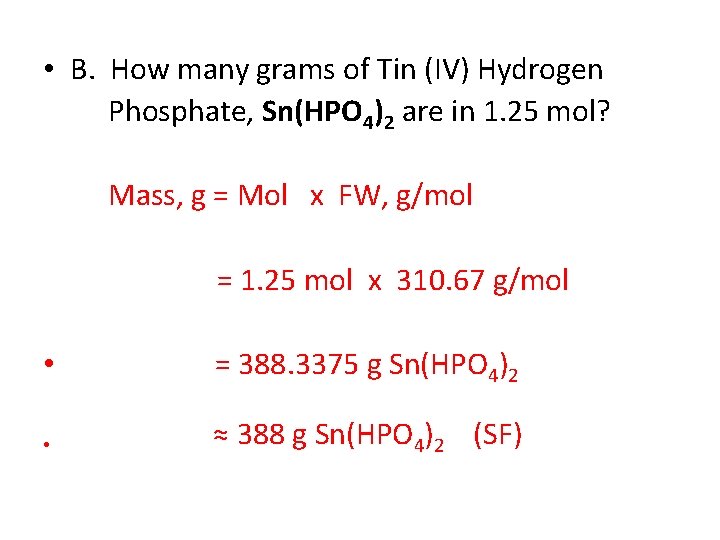
• B. How many grams of Tin (IV) Hydrogen Phosphate, Sn(HPO 4)2 are in 1. 25 mol? Mass, g = Mol x FW, g/mol = 1. 25 mol x 310. 67 g/mol • = 388. 3375 g Sn(HPO 4)2 • ≈ 388 g Sn(HPO 4)2 (SF)
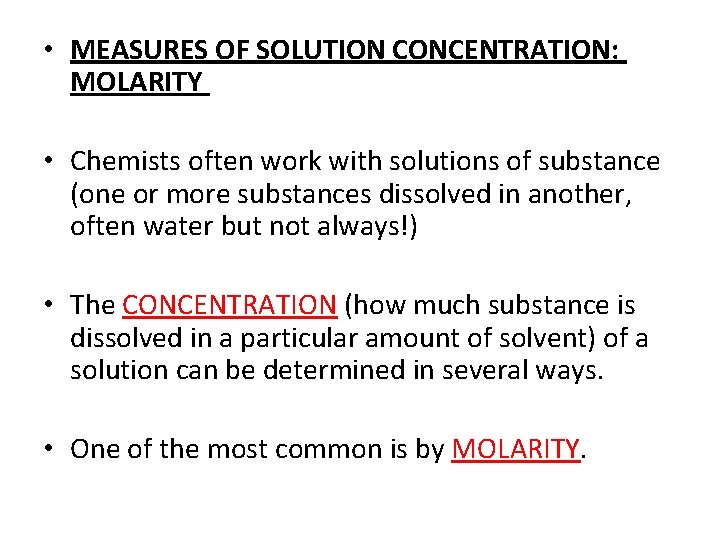
• MEASURES OF SOLUTION CONCENTRATION: MOLARITY • Chemists often work with solutions of substance (one or more substances dissolved in another, often water but not always!) • The CONCENTRATION (how much substance is dissolved in a particular amount of solvent) of a solution can be determined in several ways. • One of the most common is by MOLARITY.

• MOLARITY is MOLES OF SOLUTE per LITER OF SOLUTION. • 7. To determine the MOLARITY of a solution, two pieces of information are needed. • - First, the NUMBER OF MOLES of the SOLUTE (dissolved substance) must be known. - Second, the LITERS OF SOLUTION (solute + solvent) must be known. • • • a. The MASS OF SOLUTE is given in MOLES. b. The VOLUME OF SOLUTION is given in LITERS. If the volume is expressed in some of other volume unit, such as milliliters, m. L, or gallons, quarts, etc. , it MUST be converted to LITERS (L).

• 8. To convert m. L to L, simply divide by 1000 because there are exactly 1000 m. L in 1 Liter. • A. How many L of water are in 565 m. L? L = 565 m. L = • 1000 m. L/L • 0. 565 L (Really just moving the decimal three places to the left!) • B. To convert gallons to liters, remember that 1 gallon = 3. 79 liters. • • Liters = # gallons x 3. 79 gallons / L There are other possible volume units that you could encounter!

• 9. To calculate the MOLARITY (M) of a solution, use this equation: • • M = mol solute • liters solution
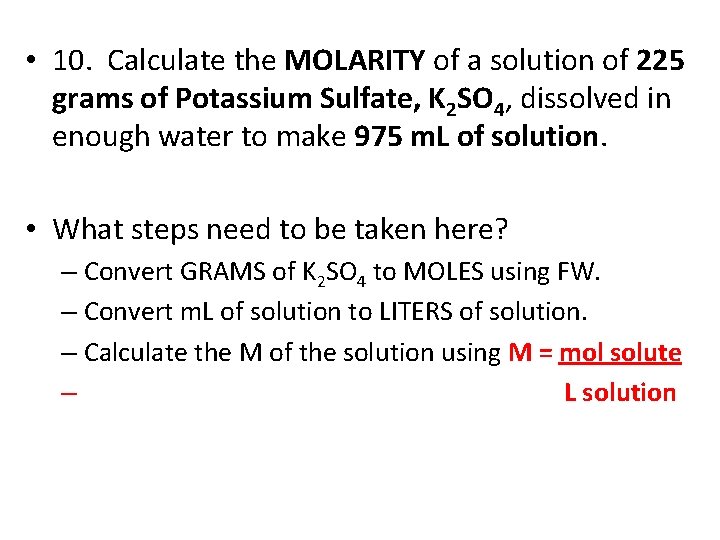
• 10. Calculate the MOLARITY of a solution of 225 grams of Potassium Sulfate, K 2 SO 4, dissolved in enough water to make 975 m. L of solution. • What steps need to be taken here? – Convert GRAMS of K 2 SO 4 to MOLES using FW. – Convert m. L of solution to LITERS of solution. – Calculate the M of the solution using M = mol solute – L solution

• FW of K 2 SO 4 = 174. 26 g/mole • Mol K 2 SO 4 = 225 g = 1. 29117 mol • 174. 26 g/mol • 975 m. L = 0. 975 L [divide by 1000 m. L/L] • M = mol solute = 1. 29117 mol K 2 SO 4 = 1. 32428 M • L solution 0. 975 L solution • ≈ 1. 32 M K 2 SO 4 (SF)

• 11. Calculate the MOLARITY of a solution of 12. 5 grams of Magnesium Hydrogen Carbonate, Mg(HCO 3)2 dissolved in enough water to make 175. 5 m. L of solution. • What steps need to be taken here? -Convert GRAMS of Mg(HCO 3)2 to MOLES using FW. -Convert m. L of solution to LITERS of solution. -Calculate the M of the solution using M = mol solute L solution
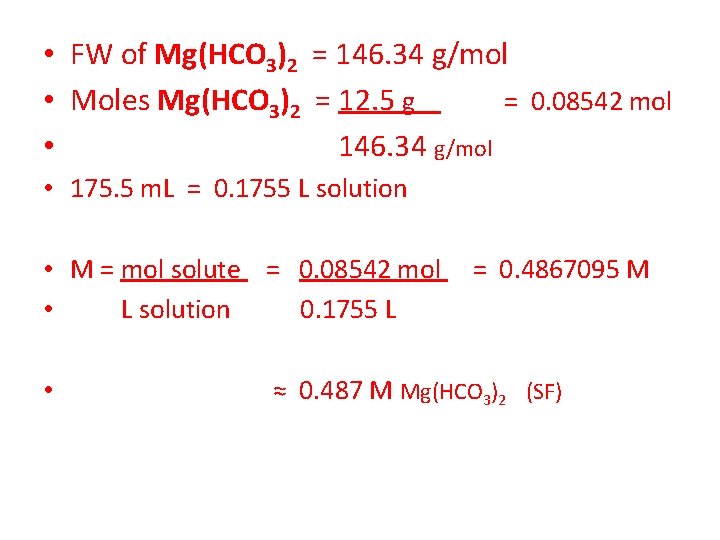
• FW of Mg(HCO 3)2 = 146. 34 g/mol • Moles Mg(HCO 3)2 = 12. 5 g = 0. 08542 mol • 146. 34 g/mol • 175. 5 m. L = 0. 1755 L solution • M = mol solute = 0. 08542 mol • L solution 0. 1755 L • = 0. 4867095 M ≈ 0. 487 M Mg(HCO 3)2 (SF)

• 12. If an aquarium requires 4. 25 gallons of salt water made from 335 grams of Table Salt, Na. Cl, what would be the MOLARITY of the aquarium’s water? • What steps need to be taken here? – -Convert GRAMS of Na. Cl to MOLES using FW. – -Convert GALLONS of solution to LITERS of solution. – -Calculate the M of the solution using M = mol solute – L solution

• FW Na. Cl = 58. 44 g/mol • Moles Na. Cl = 335 g • 58. 44 g/mol = 5. 732375 mol • 4. 25 GALLONS x 3. 79 gallons/L = 16. 1075 L • M = mol solute • L solution = 5. 732375 mol = 0. 357745 M 16. 1075 L • ≈ 0. 358 M Na. Cl (SF)

A little more of a challenge! • 13. How many GRAMS of Lithium Permanganate, Li. Mn. O 4, are contained in 575 m. L of a 0. 425 M solution? • What steps need to be taken here? • Since the question wants to know how many grams of the solute are needed to prepare a solution, we will start with M and work backward. • Once we get the # grams need to make 1 L of solution, we will use a RATIO to scale it to the amount needed for only 575 m. L of solution.

• Grams Li. Mn. O 4 in 1. 00 L of solution: • Since the M of the solution is 0. 425, that means 0. 425 moles of • solute are used to make 1. 00 L of solution, so convert 0. 425 mol of Li. Mn. O 4 to grams using its FW: mol = grams solute therefore mol x FW = g FW solute grams solute = 0. 425 mol x 125. 88 g/mol = 53. 499 grams Li. Mn. O 4 • So 53. 499 grams are needed to make 1. 00 L of solution, but the question only calls for the amount needed to make 575 m. L of solution. How do we determine this amount?
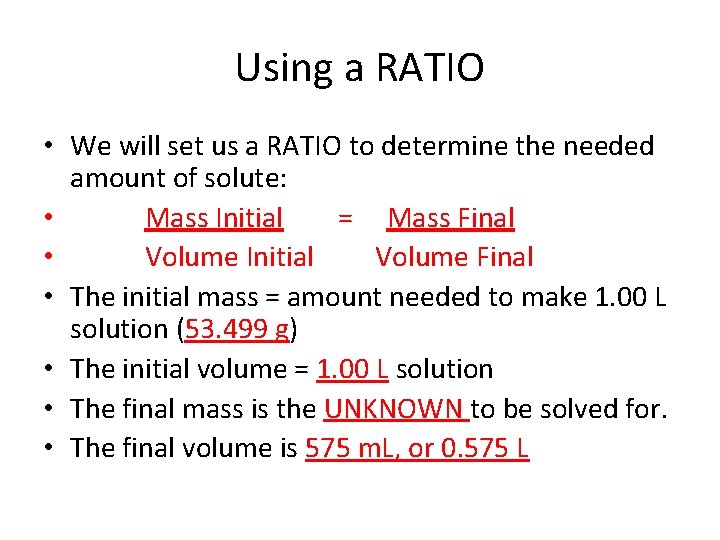
Using a RATIO • We will set us a RATIO to determine the needed amount of solute: • Mass Initial = Mass Final • Volume Initial Volume Final • The initial mass = amount needed to make 1. 00 L solution (53. 499 g) • The initial volume = 1. 00 L solution • The final mass is the UNKNOWN to be solved for. • The final volume is 575 m. L, or 0. 575 L
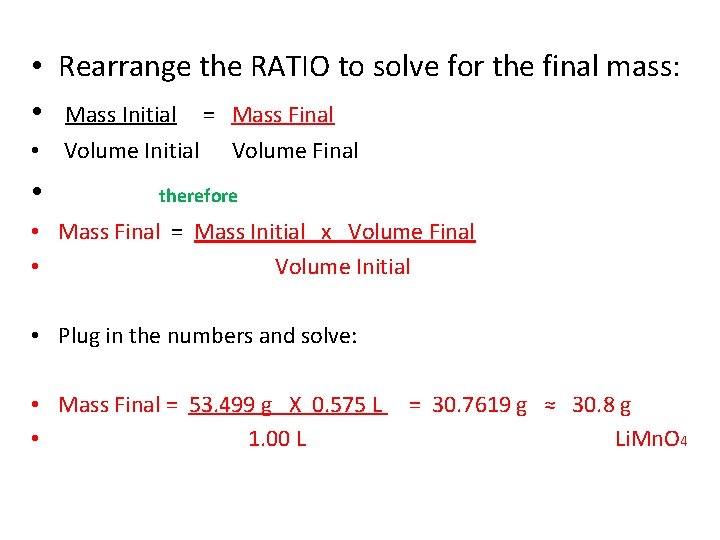
• Rearrange the RATIO to solve for the final mass: • Mass Initial = Mass Final • Volume Initial • Volume Final therefore • Mass Final = Mass Initial x Volume Final • Volume Initial • Plug in the numbers and solve: • Mass Final = 53. 499 g X 0. 575 L • 1. 00 L = 30. 7619 g ≈ 30. 8 g Li. Mn. O 4
- Slides: 24